DUBLIN-3: microtubule-binding agent plinabulin in later lines
Patients with EGFR-wildtype, advanced NSCLC in the second or third treatment line represent a large population with limited treatment options. A novel approach is the first-in-class selective immunomodulating microtubule-binding agent (SIMBA) plinabulin that releases the immune defense protein GEF-H1, thus inducing dendritic cell maturation, which is a key step in the initiation of durable anti-cancer response [1, 2]. The global, randomized phase III DUBLIN-3 trial tested plinabulin 30 mg/m2 in addition to docetaxel Q3W (n = 278) versus docetaxel plus placebo (n = 281) in patients with non-squamous or squamous, stage IIIb/IV NSCLC who had progressed during or after one or two platinum-based regimens. Overall survival constituted the primary endpoint.
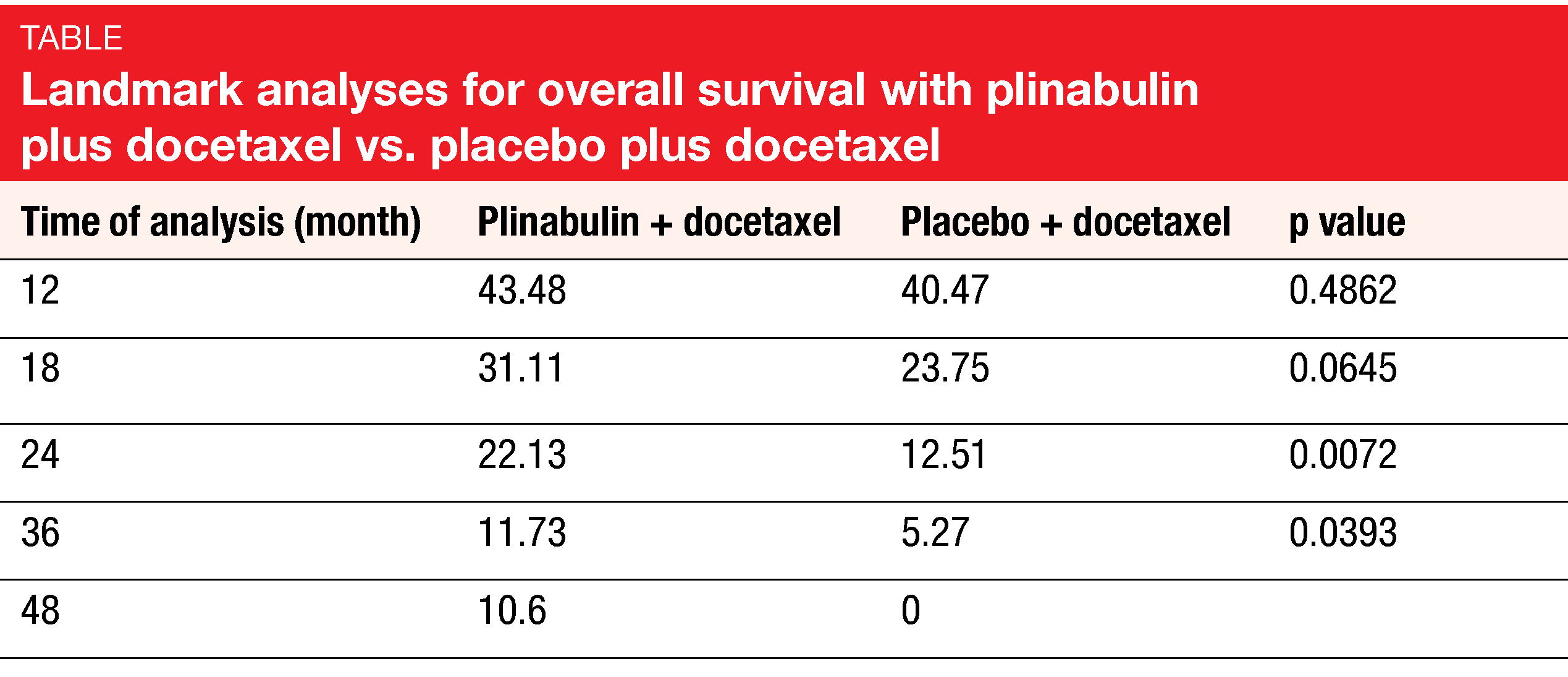
According to the results presented by Feinstein et al. at ESMO 2021, the DUBLIN-3 study met its primary objective [3]. Median OS was 10.5 vs. 9.4 months for plinabulin plus docetaxel vs. docetaxel alone (HR, 0.82; p = 0.0399). The combination gave rise to doubling of the OS rates at 24 and 36 months (Table). Likewise, the key secondary endpoints of DUBLIN-3 were met: median PFS was 3.6 vs. 3.0 months (HR, 0.76; p = 0.0082), with significantly higher PFS rates at 6 months (30.3 % vs. 17.8 %) and 12 months (17.1 % vs. 4.7 %). ORR was doubled with the addition of plinabulin (12.23 % vs. 6.76 %; p = 0.0275).
Durable activity and decreased grade 4 neutropenia
As the exploratory analysis demonstrated, the OS advantage was larger in the group that received ≥ 8 cycles (28.2 vs. 19.3 months; HR, 0.453; p = 0.0121) than in those treated with ≥ 4 cycles (18.3 vs. 13.5 months; HR, 0.634; p = 0.0022). Moreover, the analysis revealed a favorable long-term OS trend for the experimental regimen in the cohort that had been exposed to PD-(L)1-targeted therapy prior to study inclusion (i.e., 23 % of the total population), with 48-month OS rates of 12.5 % vs. 0 %. Pooled data from the phase IB/II 101 study and DUBLIN-3 suggested long-term survival benefit with the combination in Western patients.
Grade 3/4 AEs adjusted for cycle number per patient per year occurred less commonly in the experimental arm than in the control arm (estimated event rate, 9.88 vs. 11.04; p = 0.0253). Grade 4 neutropenia on day 8 in all cycles was significantly reduced with the combination (5.13 % vs. 33.58 %; p < 0.0001). Among non-hematological grade 3/4 AEs, diarrhea was reported more commonly for plinabulin plus docetaxel (8.4 % vs. 0.7 %), as was hypertension (17.2 % vs. 1.1 %) that transiently occurred after the infusion. According to the Q-TWiST analysis integrating efficacy, safety and quality of life, patients treated with the combination derived a > 18 % improvement, which is clinically meaningful. The authors concluded that plinabulin plus docetaxel shows a favorable risk-benefit ratio and has the potential of a preferred second/third-line treatment option in the setting of NSCLC with EGFR wildtype.
REFERENCES
- Kashyap AS et al., GEF-H1 signaling upon microtubule destabilization is required for dendritic cell activation and specific anti-tumor responses. Cell Rep 2019; 28(13): 3367-3380
- Singh AV et al., A novel vascular disrupting agent plinabulin triggers JNK-mediated apoptosis and inhibits angiogenesis in multiple myeloma cells. Blood 2011; 117(21): 5692-700
- Feinstein T et al., DUBLIN-3 (BPI-2358-103): a global phase 3 trial with the plinabulin/docetaxel combination vs. doc in 2nd/3rd line NSCLC patients with EGFR-wild type progressing on a prior platinum-based regimen. ESMO 2021, LBA48
© 2021 Springer-Verlag GmbH, Impressum