Immunotherapy: boosting efficacy and overcoming resistance
POSEIDON: durvalumab ± tremelimumab
The global, randomized, open-label, phase III POSEIDON trial evaluated the PD-L1 inhibitor durvalumab with or without the anti-CTLA-4 antibody tremelimumab in addition to chemotherapy as a first-line strategy in the setting of metastatic NSCLC. At 153 sites in 19 countries, 1,013 patients with squamous or non-squamous, stage IV NSCLC were randomized into three arms.One experimental arm received durvalumab 1,500 mg plus chemotherapy according to investigator’s choice Q3W for 4 cycles, which was followed by durvalumab 1,500 Q4W plus pemetrexed until progression (D+CT; n = 338). In the other experimental arm, the regimen consisted of durvalumab plus tremelimumab 75 mg and chemotherapy Q3W for 4 cycles, followed by durvalumab Q4W, tremelimumab (week 16 only) and pemetrexed until progression (D+T+CT; n = 338). Patients in the control arm received platinum-based chemotherapy Q3W for up to 6 cycles followed by pemetrexed (CT; n = 337). Johnson et al. presented the results of the study at WCLC 2021 [1].
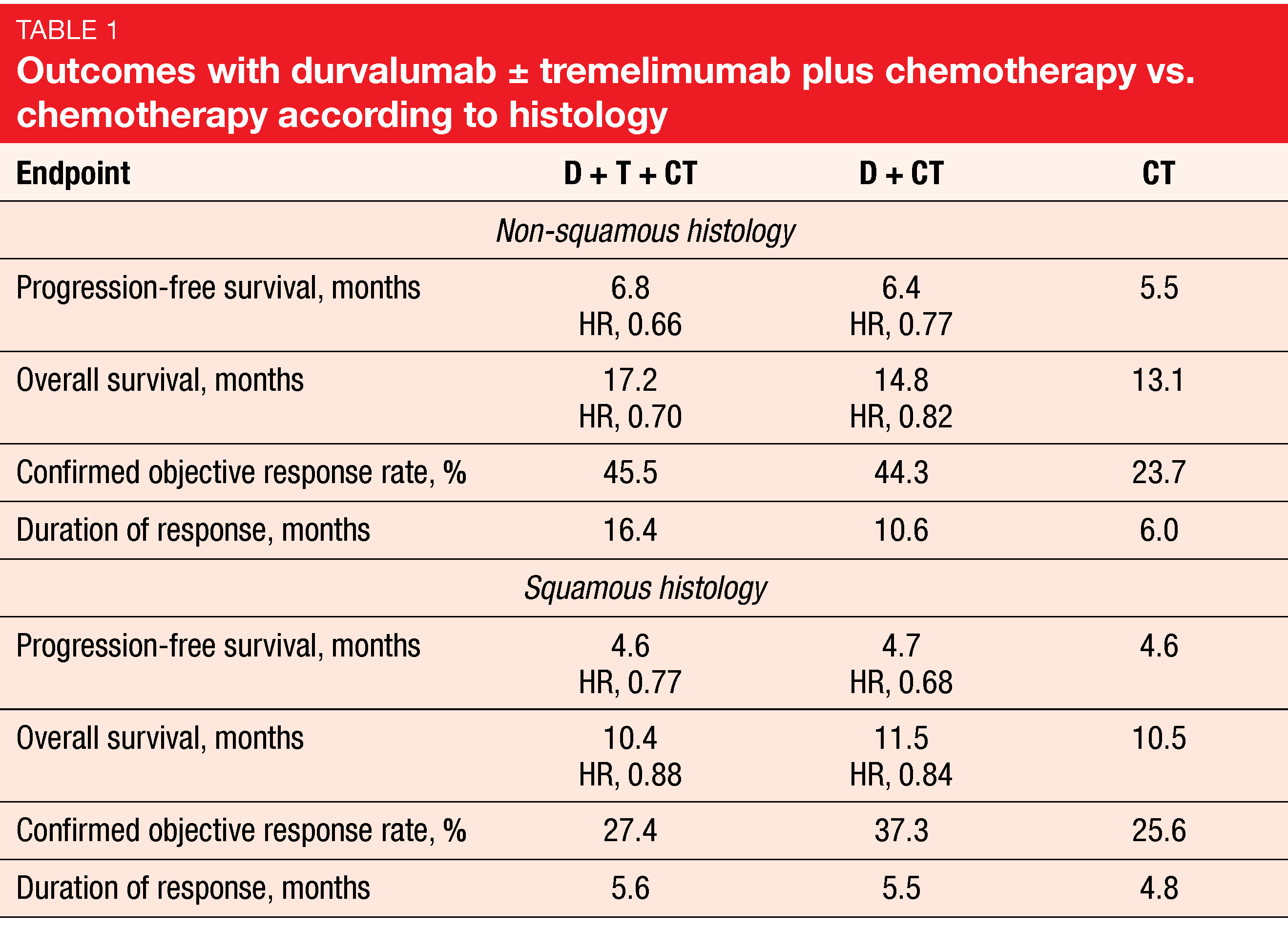
The primary endpoints were progression-free survival (PFS) and overall survival (OS) for D+CT vs. CT. Indeed, D+CT led to significant improvement of PFS compared to CT (5.5 vs. 4.8 months; HR, 0.74; p = 0.00093), while for OS, a trend favored the combination (13.3 vs. 11.7 months; HR, 0.86; p = 0.07581). The analysis of the key secondary endpoints showed that D+T+CT, as compared to CT, demonstrated statistically significant and clinically meaningful improvements in both PFS (6.2 vs. 4.8 months; HR, 0.72; p = 0.00031) and OS (14.0 vs. 11.7 months; HR, 0.77; p = 0.00304). Patients with non-squamous histology derived more prominent benefits from the triple combination than those with squamous tumors (Table 1). In the non-squamous group, the hazard ratios for PFS and OS with D+T+CT vs. CT were 0.66 and 0.70, respectively, while in the squamous group, this was 0.77 and 0.88, respectively.
Overall, the safety profile was similar across all three arms. The addition of tremelimumab to durvalumab plus CT did not give rise to a meaningful increase in the treatment discontinuation rates (15.5 % and 14.1 % with D+T+CT and D+CT, respectively). According to the authors’ conclusion, durvalumab plus tremelimumab and chemotherapy represents a potential new first-line treatment option for patients with metastatic NSCLC.
Cemiplimab plus chemotherapy: EMPOWER-Lung 3
The anti-PD-1 antibody cemiplimab has been approved as first-line monotherapy for the treatment of patients with advanced NSCLC and PD-L1 ≥ 50 % based on the EMPOWER-Lung 1 study [2]. At ESMO 2021, Gogishvili et al. reported the second interim analysis of the double-blind, randomized, phase III EMPOWER-Lung 3 trial that assessed first-line treatment with cemiplimab 350 mg plus platinum-doublet chemotherapy Q3W for 4 cycles [3]. Patients with non-squamous or squamous advanced NSCLC were randomized either to the experimental arm (n = 312) or the control arm that received placebo plus chemotherapy (n = 154). The treatment continued for up to 108 weeks or disease progression in both arms. Any PD-L1 expression was permitted.
The addition of cemiplimab to chemotherapy gave rise to clinically meaningful and statistically significant improvements in OS (21.9 vs. 13.0 months; HR, 0.71; p = 0.014), PFS (8.2 vs. 5.0 months; HR, 0.56; p < 0.0001), and objective response rate (ORR; 43.3 % vs. 22.7 %; odds ratio, 2.68; p < 0.0001). Complete remissions occurred in 2.6 % in the experimental arm, whereas there were none in the control arm. Median duration of response amounted to 15.6 vs. 7.3 months.
The combination demonstrated an acceptable benefit-risk profile with a low discontinuation rate (3 % vs. 1 %) due to treatment-related AEs (TRAEs). Any-grade immune-related AEs (irAEs) occurred in 19 %. The safety profile was generally consistent with the profiles known for cemiplimab and platinum-based chemotherapy. Patient-reported outcomes indicated delays in the time to definitive clinically meaningful deterioration in global health status/quality of life (HR, 0.78) and pain symptoms (HR, 0.39). Also, overall change from baseline in global health status/quality of life and pain symptoms improved. In their summary, the authors noted that cemiplimab in combination with platinum-doublet chemotherapy is a new first-line option for patients with advanced NSCLC without targetable mutations irrespective of histology and PD-L1 expression levels.
Post-hoc analysis of nivo/ipi in patients with brain lesions
Brain metastases are diagnosed in 10 % of patients with NSCLC and typically confer poor prognosis [4, 5]. Nivolumab in combination with ipilimumab has shown promising efficacy in patients with CNS lesions across multiple tumor types including advanced NSCLC [6-9]. The randomized phase III CheckMate 9LA trial has evaluated nivolumab 360 mg Q3W plus ipilimumab 1 mg/kg Q6W in addition to 2 cycles of chemotherapy versus 4 cycles of chemotherapy with optional pemetrexed maintenance as first-line treatment of patients with stage IV or recurrent NSCLC. According to the 2-year update, the combination provided durable survival benefit vs. chemotherapy alone, with OS rates of 38 % vs. 26 % [10].
Patients with brain metastases that were adequately treated and asymptomatic for ≥ 2 weeks prior to the first dose were allowed to participate. In the experimental and control arms, this group included 51 and 50 individuals, respectively, while 310 and 308, respectively, had no brain lesions at baseline. At WCLC 2021, Carbone et al. reported a post-hoc analysis of patients with and without brain metastases after a minimum follow-up of 2 years [11].
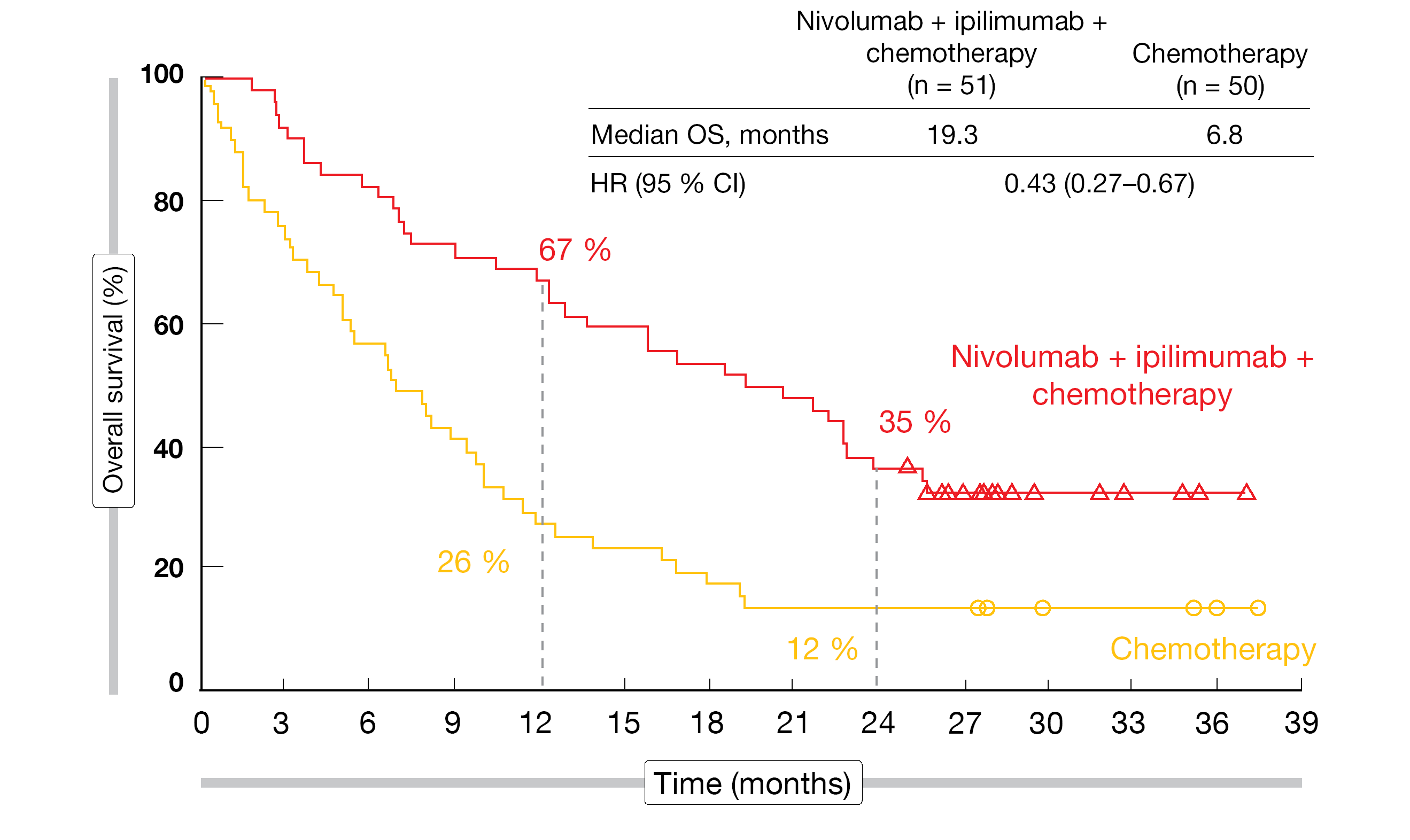
The findings showed that nivolumab plus ipilimumab in addition to chemotherapy induced significant survival benefit compared to chemotherapy alone independent of the presence of baseline brain metastases. Patients with CNS lesions achieved an even more pronounced mortality reduction (median OS, 19.3 vs. 6.8 months; HR, 0.43; Figure 1) than those without (15.6 vs. 12.1 months; HR, 0.79). Likewise, regarding systemic response, the HRs were 0.40 and 0.74 for the two cohorts. The ORRs were 43 % and 37 % in the experimental arms of the groups with and without brain lesions, respectively, and median duration of response was 15.5 and 13.0 months, respectively.
Among the patients with CNS metastases, nivolumab plus ipilimumab and chemotherapy improved intracranial efficacy in terms of PFS (13.5 vs. 4.6 months; HR, 0.36), ORR (39 % vs. 20 %), and duration of response (22.3 vs. 18.9 months), which was consistent with systemic efficacy. Complete intracranial responses resulted in 10 % vs. 8 %. Fewer patients developed new brain metastases with the immunotherapy-based approach, both in the group with baseline CNS lesions (16 % vs. 30 %) and those without (2 % vs. 4 %). Median time to development of new brain metastases was 9.0 vs. 4.6 months in the patients who had baseline brain lesions. In their summary, the authors noted that these data further support the use of nivolumab plus ipilimumab in addition to chemotherapy as an efficacious first-line treatment option in patients with advanced NSCLC, including those with brain metastases.
Figure 1: CheckMate 9LA: overall survival with nivolumab plus ipilimumab and chemotherapy vs. chemotherapy only in patients with baseline brain metastases
ATEZO-BRAIN
The single-arm, phase II, Bayesian ATEZO-BRAIN trial aimed to determine the activity and safety of atezolizumab in patients with stage IV non-squamous NSCLC and untreated brain metastases based on the observation that this patient group is underrepresented in clinical trials evaluating chemotherapy plus immunotherapy in the first-line setting. Forty patients received carboplatin (5 AUC) plus pemetrexed 500 mg/m2 and atezolizumab 1,200 mg Q3W for 4-6 cycles followed by pemetrexed plus atezolizumab Q3W for a maximum of 2 years. Anticonvulsants and dexamethasone at ≤ 4 mg daily doses were permitted. Safety and PFS constituted the co-primary endpoint.
Atezolizumab plus carboplatin and pemetrexed demonstrated a favorable safety profile and efficacy in these patients, including those receiving corticosteroids [12]. The trial was completed as the boundaries for futility or unacceptable toxicity were not reached, with a 12-week PFS rate of 60 % and a grade 3-4 toxicity rate of 27.5 %. Most TRAEs were grade 1 and 2, and no fatal TRAEs occurred. Three patients had grade 4 TRAEs including thrombocytopenia, neutropenia, and hallucinations. Among AEs in general, fatigue (any grade, 60 %) and anemia (45 %) were observed most commonly. Selected immune-related AEs mainly comprised skin rash (20 %) and increases of the transaminases (13 %). One case of grade 3 pneumonitis was reported (3 %).
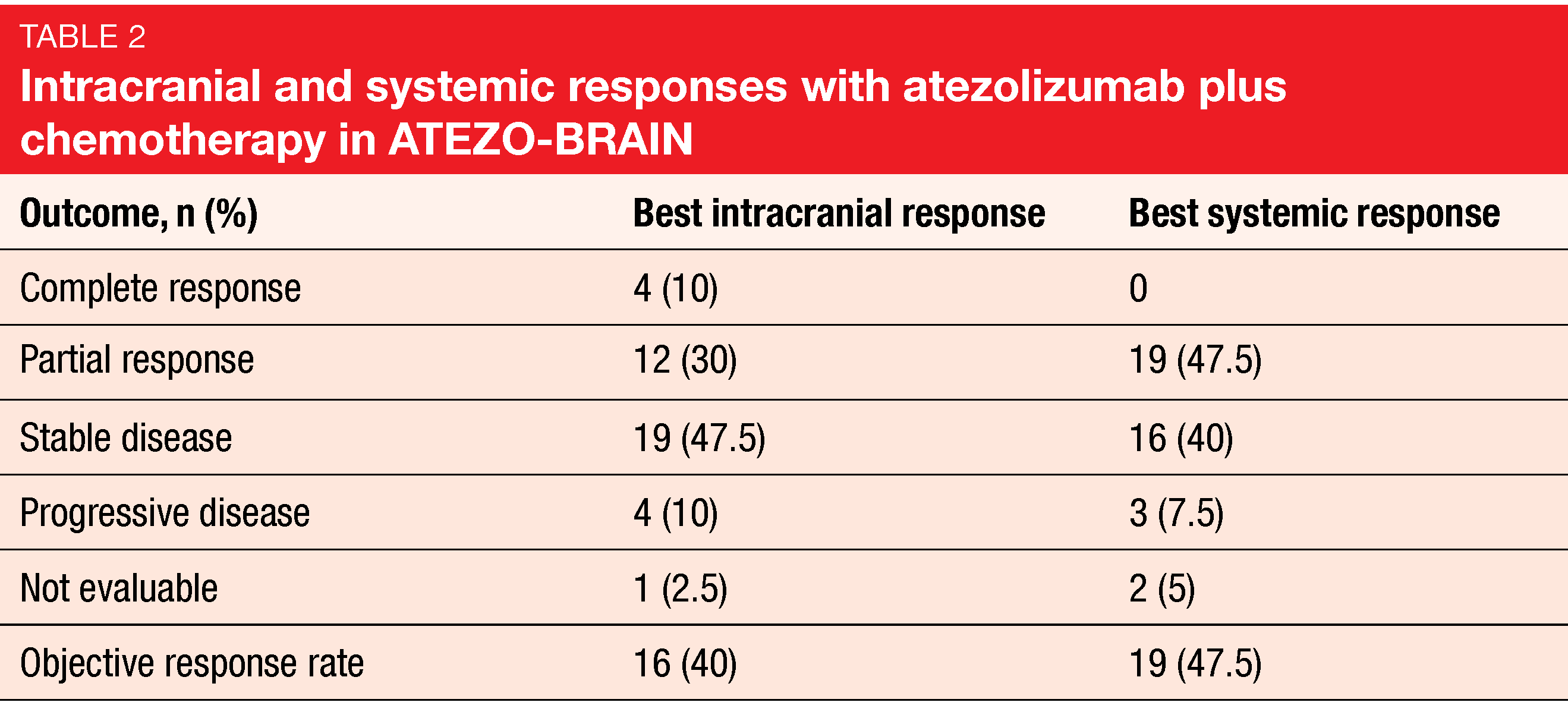
Median systemic PFS was 8.9 months, and 24.9 % of patients were progression-free at 18 months. Median intracranial PFS amounted to 6.9 months, which is similar to the PFS reported in the KEYNOTE-189 trial in patients with brain metastases [13]. Most responses in body and brain were concordant (Table 2). Forty and 47.5 % of patients achieved intracranial and systemic responses, respectively. Median OS was 13.6 months, and at 2 years, 32 % were alive. Correlative studies based on brain imaging and blood samples are ongoing.
MRTX-500: sitravatinib plus nivolumab
Although checkpoint inhibitor therapy has changed the treatment landscape for NSCLC, resistance through various mechanisms including the development of an immunosuppressive tumor microenvironment is common, and treatment options are limited in the setting of disease refractory/resistant to anti-PD-(L)1 therapies. It was hypothesized that the combination of nivolumab with the tyrosine kinase inhibitor sitravatinib is a rational approach to augmenting the anti-tumor immune response and extending long-term patient benefit. Sitravatinib targets the TAM receptors TYRO3, AXL, MERTK, as well as VEGFR2 and KIT that have been shown to reduce myeloid-derived suppressor cells and enhance anti-tumor immune responses, among others [14]. The open-label, single-arm, phase II MRTX-500 trial tested sitravatinib 120 mg once daily plus nivolumab in patients with non-squamous, advanced NSCLC who had benefited from anti-PD-(L)1 therapy (i. e., complete or partial response or stable disease ≥ 12 weeks). Checkpoint inhibitors had been administered as the most recent line of treatment.
At ESMO 2021, Leal et al. reported updated efficacy and safety of sitravatinib plus nivolumab in the second- or third-line settings [15]. This analysis comprised 68 patients. The ORR, which constituted the primary endpoint, was 18 %, including complete remissions in 3 %. Seventy-eight percent of patients obtained disease control, and responses lasted for a median of 12.8 months. Median PFS and OS were 5.7 and 14.9 months, respectively. The 24-month OS rate was 32 %.
Among TRAEs, diarrhea (all grades, 62 %), fatigue (52 %) and nausea (44 %) were reported most commonly. The most frequent immune-related TRAEs included hypothyroidism, diarrhea, transaminase elevations, TSH increase, maculopapular rash, and pancreatitis. No grade 5 events occurred. TRAEs prompted treatment discontinuation in 22 %. In 81 %, at least one dose interruption of sitravatinib had to be performed due to AEs. These results support the ongoing randomized, open-label phase III SAPPHIRE trial assessing second-/third-line sitravatinib plus nivolumab versus docetaxel after progression on or following checkpoint inhibition in patients with advanced NSCLC.
Tislelizumab as combination partner of sitravatinib
The anti-PD-1 antibody tislelizumab has been designed to minimize binding to FcγR on macrophages in order to abrogate antibody-dependent phagocytosis, which is a mechanism of T cell clearance and anti-PD-1 resistance [16, 17]. Tislelizumab is being investigated in combination with sitravatinib in several solid tumor types with the aim of enhancing anti-tumor activity beyond that provided by either agent alone. An open-label, multicenter, non-randomized phase Ib trial tested sitravatinib 120 mg once daily plus tislelizumab 200 mg Q3W. Zhou et al. presented the results for Cohorts A, B and F that contained patients with squamous or non-squamous metastatic NSCLC who had received 1–3 prior lines of systemic therapy with or without an anti-PD-(L)1 inhibitor (n = 75) [18]. Any PD-L1 expression level was permitted.
The findings suggested efficacy of the combination irrespective of checkpoint inhibitor pretreatment. The overall ORR was 16.9 %, with a numerically higher proportion of anti-PD-(L)1-naïve patients responding compared to the relapsed/refractory cohort (22.2 % and 13.6 %, respectively). Likewise, anti-PD-(L)1-naïve patients experienced numerically longer PFS (7.0 months) and OS (15.3 months) than the pretreated group (PFS, 5.2 months; OS, 10.1 months). In the total cohort, median PFS and OS were 5.5 months and 11.9 months, respectively. Disease control was achieved in 84.5 %. Tislelizumab plus sitravatinib demonstrated a manageable safety profile consistent with what had previously been reported. Hypertension was the most commonly reported grade ≥ 3 treatment-emergent AE and TRAE, although no cases of hypertension led to treatment discontinuation.
Gao et al. presented a separate analysis of Cohorts A and F that included 47 patients with squamous and non-squamous metastatic NSCLC refractory or resistant to anti-PD-(L)1 therapy [19]. Disease control resulted in 86.4 % in this group, with median duration of response of 6.9 months. According to the authors, the promising anti-tumor activity observed in this study supports sitravatinib plus tislelizumab as a potential treatment option for patients with metastatic NSCLC that is relapsed or refractory to prior anti-PD-(L)1 therapy. Further investigation is warranted.
Results according to smoking status in RATIONALE 304 & 307
In the RATIONALE 304 study, tislelizumab plus chemotherapy gave rise to significantly improved PFS compared to chemotherapy alone as first-line treatment of patients with advanced non-squamous NSCLC [20]. According to the sub-analysis presented by Lu et al. at ESMO 2021, the efficacy and safety of tislelizumab plus chemotherapy in smokers (63.8 % of the population) was consistent with the findings observed in the overall population [21]. Median PFS for the two treatment arms was 9.7 vs. 4.6 months (HR, 0.466) in smokers, while these results did not differ in non-smokers (8.5 vs. 7.7 months; HR, 1.075). Tislelizumab in addition to chemotherapy induced higher ORR regardless of smoking status.
A similar sub-analysis of the RATIONALE 307 study that investigated first-line tislelizumab plus chemotherapy in NSCLC patients with squamous histology suggested consistent PFS and ORR independent of smoking status [22]. The primary analysis of RATIONALE 307 had revealed a significant PFS benefit and manageable safety compared with chemotherapy alone [23]. In this study, 83.7 % of patients were smokers. This group showed a median PFS of 7.6 months with both chemotherapy regimens (i.e., paclitaxel or nab-paclitaxel plus carboplatin) vs. 5.5 months with chemotherapy alone. Non-smokers equally benefited from the addition of tislelizumab in terms of PFS, with HRs of 0.475 and 0.119 for the two treatment regimens. ORR was higher in the experimental arm regardless of smoking status. In both RATIONALE 304 and 307, the safety profile recorded in smokers and non-smokers was consistent with that observed in the overall population.
STK11/KRAS co-mutations: predictive significance
Basher et al. elucidated the predictive role of STK11/KRAS co-mutations identified by next-generation sequencing and the incidence of irAEs in the setting of immune checkpoint inhibitor treatment [24]. Overall, 703 patients with stage IIIB/IV NSCLC from three centers in Florida were retrospectively analyzed.
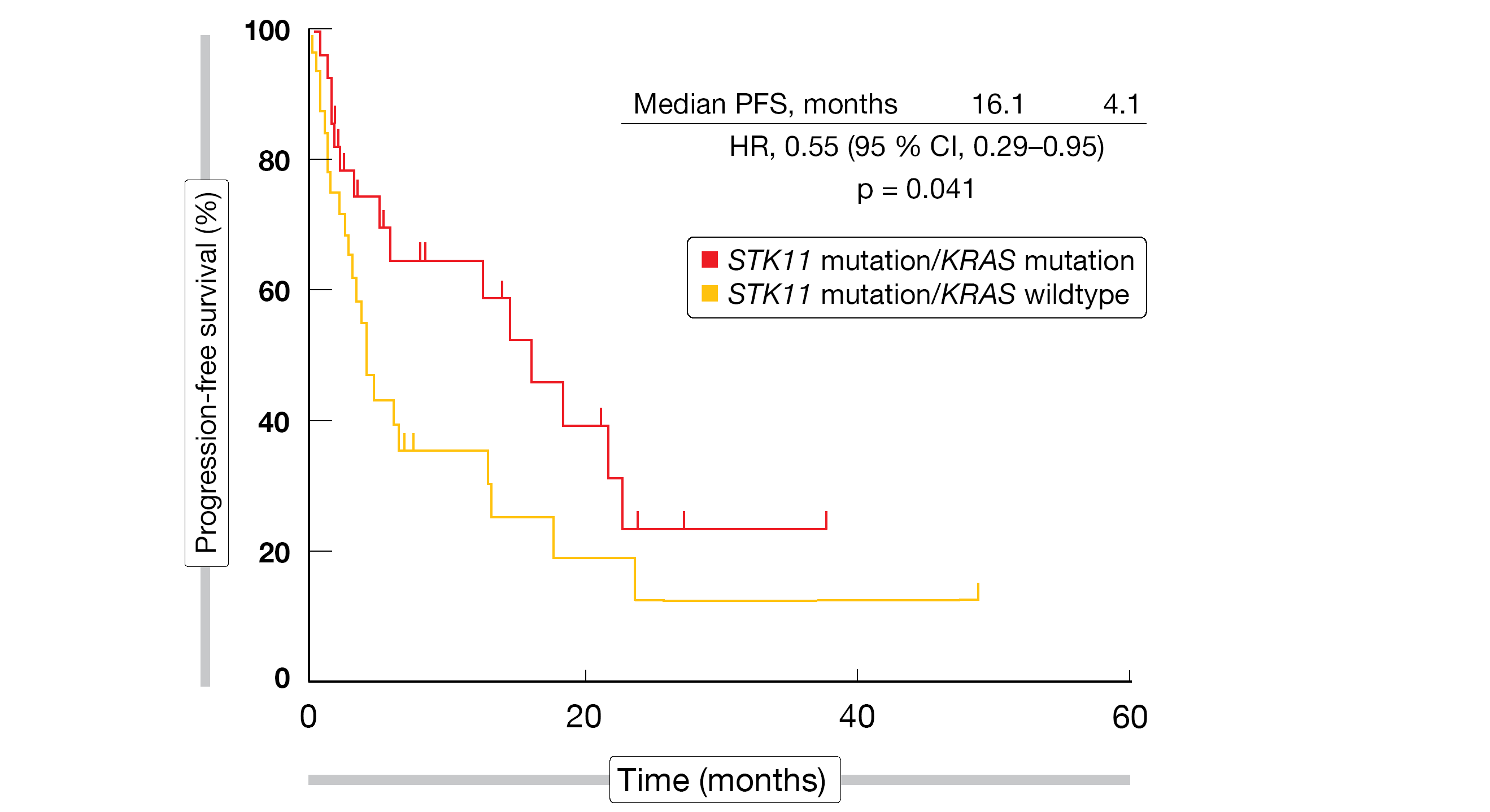
Indeed, concomitant STK11 and KRAS mutation was associated with significantly improved PFS compared to STK11 mutation/KRAS wildtype (16.1 vs. 4.1 months; HR, 0.55; p = 0.041; Figure 2). For OS, the analysis showed a trend favoring the co-mutation (32.3 vs. 21.8 months; HR, 0.61; p = 0.21). irAEs were shown to predict OS improvement with the treatment: patients who developed irAEs lived for a median of 46.3 months compared to 29.7 months in the cohort without immune-related side effects (HR, 0.59; p = 0.022). While Hispanic patients experienced irAEs more frequently than their non-Hispanic white counterparts, they did not derive a significant survival benefit. Further studies might identify unique aspects of this population that can account for these observations.
Figure 2: Significantly prolonged progression-free survival on immune checkpoint inhibition in the presence of STK11/KRAS co-mutations
REFERENCES
- Johnson ML et al., Durvalumab ± tremelimumab + chemotherapy as first-line treatment for mNSCLC: results from the phase 3 POSEIDON study. WCLC 2021, PL02.01
- Sezer A et al., Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 2021; 397(10274): 592-604
- Gogishvili M et al., EMPOWER-Lung 3: cemiplimab in combination with platinum-doublet chemotherapy for first-line treatment of advanced non-small cell lung cancer. ESMO 2021, LBA51
- Waqar NS et al., Non-small-cell lung cancer with brain metastasis at presentation. Clin Lung Cancer 2018; 19(4): e373-e379
- Sperduto PW et al., Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol 2017; 3(6): 827-831
- Di Giacomo AM et al., Immunotherapy of brain metastases: breaking a “dogma”. J Exp Clin Cancer Res 2019; 38(1): 419
- Tawbi HA et al., Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med 2018; 379(8): 722-730
- Emamekhoo H et al., Safety and efficacy of nivolumab plus ipilimumab (NIVO+IPI) in patients with advanced renal cell carcinoma (aRCC) with brain metastases: Interim analysis of CheckMate 920. J Clin Oncol 37, 2019 (suppl; abstr 4517)
- Borghaei H et al., Nivolumab + ipilimumab as first-line treatment for patients with advanced non-small cell lung cancer with brain metastases: results from Checkmate 227. Cancer Research 2020; 80(Suppl. 16): Abstract CT221
- Reck M et al., First-line nivolumab plus ipilimumab plus two cycles of chemotherapy versus chemo alone (4 cycles) in patients with advanced non-small cell lung cancer: Two-year update from CheckMate 9LA. J Clin Oncol 39, 2021 (suppl 15; abstr 9000)
- Carbone DP et al., First-line nivolumab + ipilimumab + chemotherapy in patients with advanced NSCLC and brain metastases: results from CheckMate 9LA. WCLC 2021, OA09.01
- Nadal E et al., ATEZO-BRAIN (GECP 17/05): non-randomized phase II clinical trial of atezolizumab combined with carboplatin plus pemetrexed in chemotherapy-naïve patients with advanced non-squamous NSCLC with untreated brain metastases. WCLC 2021, OA09.02
- Garassino MC et al., Outcomes among patients with metastatic nonsquamous NSCLC with liver metastases or brain metastases treated with pembrolizumab plus pemetrexed-platinum: Results from the KEYNOTE-189 study. AACR Annual Meeting 2019, CT043
- Du W et al., Sitravatinib potentiates immune checkpoint blockade in refractory cancer model. JCI Insight 2018; 3: e124184
- Leal TA et al., MRTX-500: phase 2 trial of sitravatinib + nivolumab in patients with nonsquamous non-small-cell lung cancer progressing on or after prior checkpoint inhibitor therapy. ESMO 2021, 1191O
- Qin S et al., RATIONALE 301 study: tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol 2019; 15: 1811-1822
- Zhang T et al., The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother 2018; 67(7): 1079-1090
- Zhou Q et al., Sitravatinib + tislelizumab in patients with metastatic non-small cell lung cancer. ESMO 2021, 1280P
- Gao B et al., Sitravatinib + tislelizumab in patients with anti-PD-(L)1 refractory/resistant metastatic non-small cell lung cancer. ESMO 2021, 1284P
- Lu S et al., Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): a randomized phase 3 trial. J Thorac Oncol 2021; 16(9): 1512-1522
- Lu S et al., RATIONALE 304: tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for non-squamous non-small cell lung cancer in patients who are smokers vs non-smokers. ESMO 2021, 1290P
- Yu X et al., RATIONALE 307: tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small cell lung cancer in patients who were smokers vs non-smokers. ESMO 2021, 1297P
- Wang J et al., Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol 2021; 7(5): 709-717
- Basher F et al., Prognostic value of STK11 & KRAS mutations and irAE incidence in response to immunotherapy in Hispanics: a multicenter analysis. WCLC 2021, MA01.07
© 2021 Springer-Verlag GmbH, Impressum