PD-1 inhibition in gastric and esophageal cancer, hepatocellular carcinoma, and urothelial carcinoma
Gastric and esophageal cancer
Adenocarcinoma of the stomach and gastroesophageal junction (GEJ) ranks fifth among the most common malignancies worldwide [1, 2] and is the third leading cause of cancer-related death in both sexes [3]. Most patients are diagnosed at an advanced stage due to the asymptomatic early feature of the disease. Despite a falling global incidence and significant progress in treatment, further efforts are necessary to improve prognosis [4].
Esophageal cancer is the seventh most common cancer worldwide [5]. In 2018, it ranked fifth in incidence and fourth in mortality of all cancer types in China [6]. The most common subtypes are squamous cell carcinoma (SCC) and adenocarcinoma (AC) [5, 7]. These entities show differences in etiology and prevalence across countries. Metastatic esophageal cancer has a poor prognosis, with a 5-year relative survival rate of ≤ 8 % [8, 9]. In both gastric/GEJ and esophageal cancer, systemic treatment in the advanced setting includes chemotherapy in the first- and second-line settings plus anti-HER2 tumors in first line, as well as the anti-VEGFR-antibody ramucirumab in second line. However, chemotherapy efficacy is limited, with substantial toxicity. The armamentarium is currently being expanded by PD-1 inhibitors, which are successfully tested in clinical trials.
Second-line nivolumab in esophageal SCC
The randomized, open-label, phase III ATTRACTION-3 study assessed nivolumab in patients with unresectable advanced or recurrent esophageal SCC who were refractory to or intolerant of one prior fluoropyrimidine/platinum-based chemotherapy. Patients in the experimental arm received nivolumab monotherapy (n = 210), while those in the control arm were treated with either docetaxel or paclitaxel (n = 209). Overall survival (OS) constituted the primary endpoint.
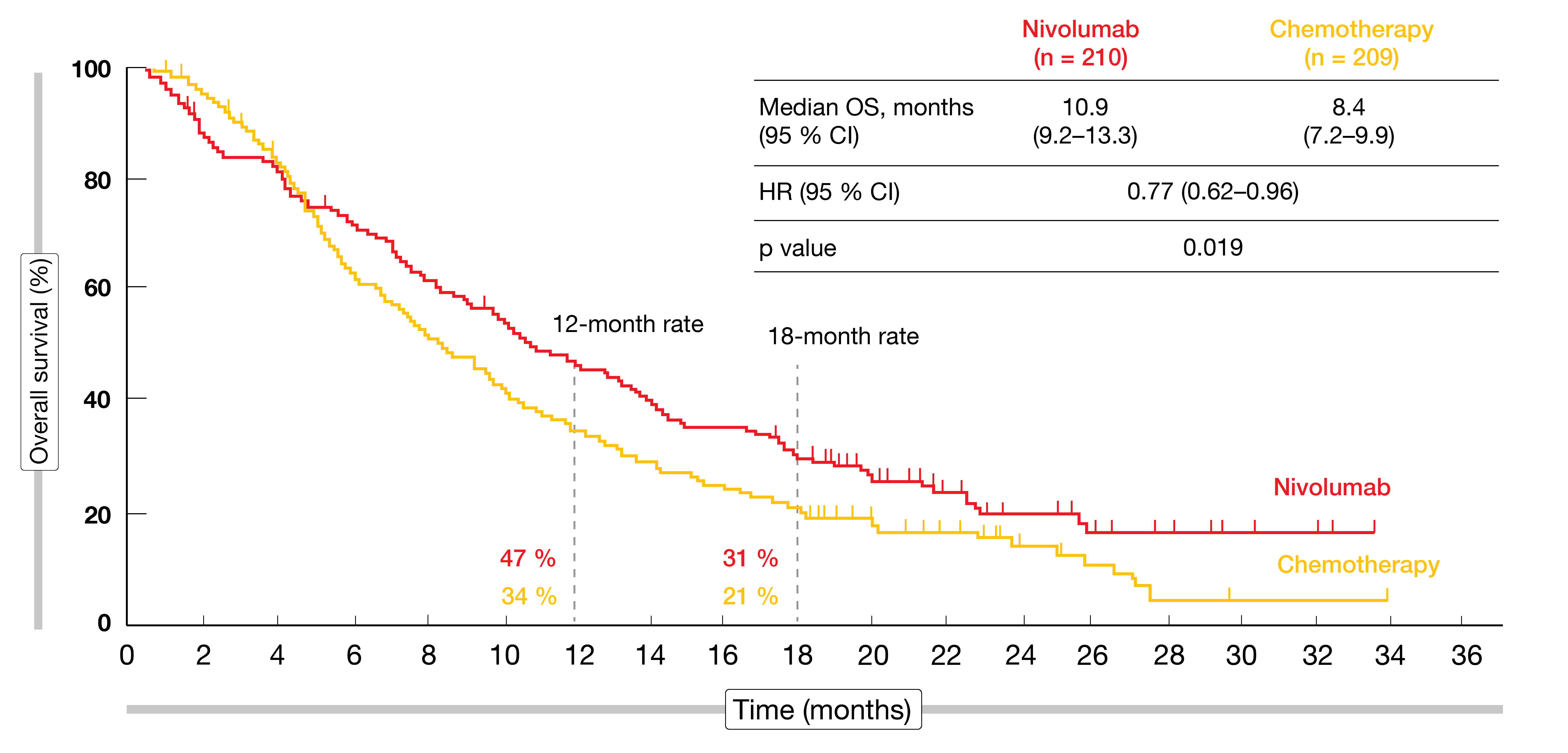
According to the final analysis reported at ESMO 2019, nivolumab demonstrated a statistically significant and clinically meaningful improvement in OS compared to chemotherapy in pretreated advanced esophageal SCC, with a 23 % reduction in mortality risk (10.9 vs. 8.4 months; HR, 0.77; p = 0.019; Figure 1) [10]. Eighteen-month OS rates were 31 % vs. 21 % for the two arms. The subgroup analysis consistently favored nivolumab across various pre-specified subgroups, which also included PD-L1 expression. No meaningful difference between nivolumab and chemotherapy was achieved regarding progression-free survival (PFS; HR, 1.08). Also, the two treatment regimens gave rise to comparable objective response rates (ORRs; 19 % vs. 22 %), although, notably, responses proved more durable in the nivolumab arm (6.9 vs. 3.9 months).
Health-related quality of life (HRQol) was assessed using the EQ-5D-3L visual analog scale score. According to this exploratory analysis, the nivolumab-based treatment elicited significant overall improvement. This might also have been due to the lower rate of treatment-related adverse events (TRAEs) reported for nivolumab. Moreover, the PD-1 inhibitor gave rise to a greater percentage of low-grade AEs compared to high-grade AEs, whereas the opposite was the case for chemotherapy. The incidence of grade 3/4 AEs was more than 3 times higher in the control arm (18 % vs. 63 %). Grade 3/4 select TRAEs including endocrine, gastrointestinal, pulmonary and renal events occurred in ≤ 2 % of patients in both arms. In their conclusion, the authors stated that nivolumab represents a potential new standard second-line option for patients with advanced esophageal SCC.
Figure 1: Superiority of nivolumab vs. chemotherapy regarding overall survival in esophageal squamous-cell carcinoma (ATTRACTION-3 study)
Gastric cancer: real-world results with nivolumab
The benefit of nivolumab in pretreated patients with gastric or GEJ cancer has been established by the phase III ATTRACTION-2 study [11]. At ESMO 2019, Sunakawa et al. presented real-world data on the use of single-agent nivolumab in any line in 198 patients with advanced gastric or GEJ cancer [12]. In addition, the researchers investigated the association of outcomes with host-related factors. Ninety-two and 80 % of patients had already received taxanes and ramucirumab, respectively. Peritoneal metastases and ascites were present in approximately half of the cases.
Among 119 of patients with measurable lesions, 5.6 % responded to treatment, and disease control was achieved in 33.1 %. A subanalysis by patient background indicated that disease control rates (DCR) were 38 % for PS 0, 35 % for PS 1, and 22 % for PS 2. Also, the DCR was lower in those with peritoneal metastasis and ascites, as well as in patients with signet-ring cell histology compared to other histological subtypes. HER2 mutation status and neutrophil-lymphocyte ratio, on the other hand, did not affect disease control.
In 58.4 % of 105 evaluable patients, the tumor growth rate (i.e., the increase in tumor volume during 1 month) decreased after the introduction of nivolumab. Twenty-six (24.8 %) were identified as patients with hyper-progressive disease (HPD), i.e. those with a ≥ 2-fold increase of the tumor growth rate during nivolumab as compared to before nivolumab. According to a subanalysis, HPD occurred more commonly in patients with body mass index ≥ 25 (40.0 % vs. 24.0 % in those with BMI < 25) and peritoneal metastases (33.3 % vs. 20.8 % in those without). Moreover, compared to patients who had not received the respective agents, HPD showed higher incidence after previous use of taxanes (25.5 % vs. 14.3 %) and irinotecan (40.0 % vs. 21.1 %). Age, PS, HER2 status and history of use of antibiotics did not correlate with the HPD rate. None of the patients with signet-ring cell histology experienced HPD.
KEYNOTE-181: pembrolizumab vs. chemotherapy in Chinese patients
Single-agent pembrolizumab was assessed in the global, randomized, phase III KEYNOTE-181 trial that included patients with advanced AC or SCC of the esophagus or Siewert type 1 AC of the GEJ, who had progressed during or after first-line therapy. The control arm received chemotherapy including paclitaxel, docetaxel, or irinotecan according to investigator’s choice. In each arm, 314 patients were treated. The final analysis of the trial reported by Kojima et al. showed that pembrolizumab substantially improved OS over chemotherapy in patients with PD-L1 combined positive score (CPS) ≥ 10 [13].
Given the differences in disease etiology between SCC and AC and the overwhelming prevalence of SCC in Chinese patients, Chen et al. focused on the results obtained in the Chinese subgroup of KEYNOTE-181 (n = 123) [14]. Sixty-two and 61 of these patients received pembrolizumab and chemotherapy, respectively. Almost the entire cohort had SCC histology (97 % in each treatment arm). PD-L1 CPS ≥ 10 was present in 40.3 % and 47.5 % in the pembrolizumab and chemotherapy arms, respectively.
Meaningful OS activity irrespective of PD-L1 status
The primary end point was OS in patients with PD-L1 CPS ≥ 10 (n = 54), those with SCC histology (n = 119), and the total population (ITT; n = 123). Indeed, pembrolizumab resulted in survival improvement compared to chemotherapy for all of these cohorts, with HRs amounting to 0.34, 0.55, and 0.55. Twelve-month OS rates for pembrolizumab versus chemotherapy in the three groups were 53.1 % vs. 16.1 %, 35.7 % vs. 15.3 %, and 36.3 % vs. 16.7 %. For PFS, which was defined as a secondary endpoint, pembrolizumab showed no superiority, with similar 6-month rates in the two treatment arms. Objective response rates in the experimental arm substantially exceeded those observed in the control arm in all three groups. Overall, median duration of response had not been reached yet for pembrolizumab and was 3.2 months for chemotherapy. At the same time, the PD-1 inhibitor demonstrated improved tolerability, with fewer patients experiencing any-grade, grade 3-5 TRAEs or TRAEs that resulted in discontinuation. Hypothyroidism, ALT increases and asthenia ranged among the most common AEs.
In their conclusions, the authors noted that although most patients in the Chinese cohort had SCC histology, pembrolizumab showed clinically meaningful OS improvement regardless of PD-L1 status. This was consistent with the OS prolongation noted in the Asian subgroup of the full global cohort. Overall, these results suggested that pembrolizumab could be a novel standard-of-care agent in the second-line treatment of Chinese patients with advanced esophageal cancer.
HRQoL in KEYNOTE-061
Patients with advanced adenocarcinoma of the stomach or GEJ and PD-L1 CPS ≥ 1 received second-line treatment with either pembrolizumab or paclitaxel in the randomized, multicenter, open-label, phase III KEYNOTE-061 trial. Here, the primary analysis had not yielded any significant differences in terms of OS or PFS [15]. However, pembrolizumab therapy induced more durable responses and a better safety profile than paclitaxel. Van Cutsem et al. reported results of pre-specified exploratory HRQoL analyses conducted in the primary analysis population from KEYNOTE-061 [16]. Changes from baseline HRQoL were assessed using the EORTC QLQ-C30 and EORTC QLQ-STO22 questionnaires. For the characterization of health status, EuroQol EQ-5D-3L was used. The HRQoL population included 371 patients.
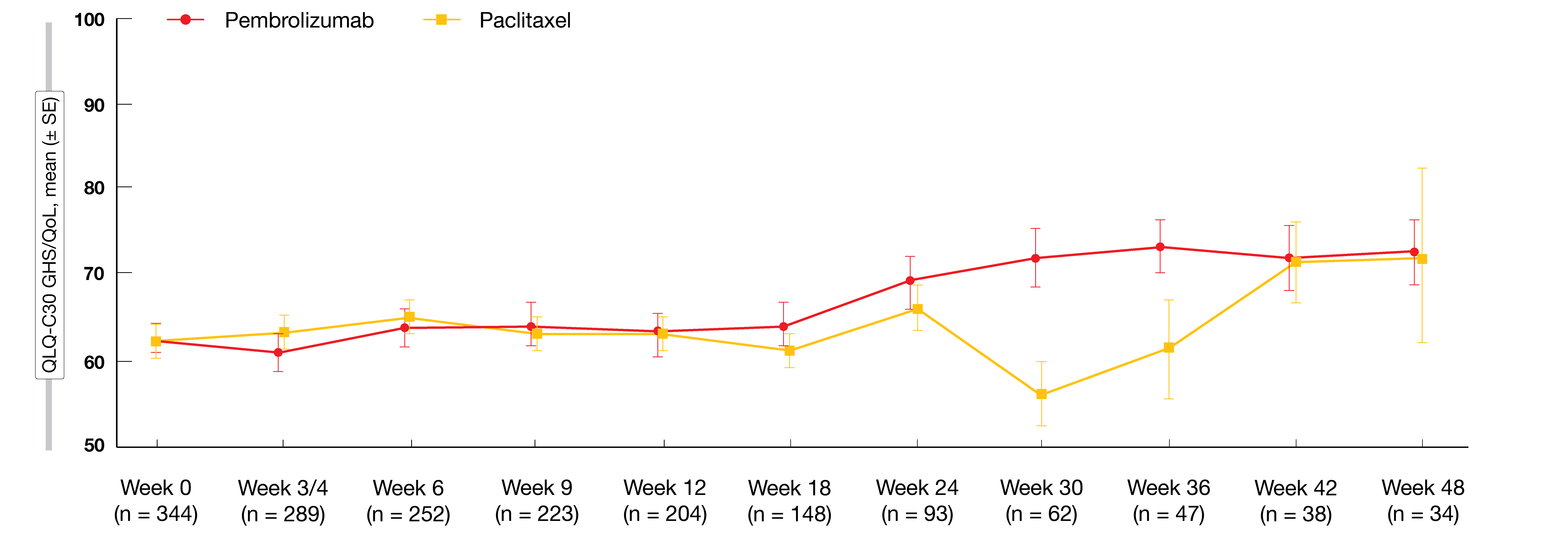
Global health status/QoL scores worsened over the first 12 weeks in both treatment groups but improved in the experimental arm compared to the control arm from week 18 (Figure 2). Median time to deterioration in QLQ-C30 and QLQ-STO22 scores was similar for pembrolizumab and paclitaxel, which also applied to the pre-specified nausea/vomiting and appetite loss subscales in QLQ-C30 and the pain subscale in QLQ-STO22. The authors noted that together with previously presented efficacy and safety data, these findings from the KEYNOTE-061 study underscore the need for further research to identify patients likely to benefit from single-agent pembrolizumab.
Figure 2: KEYNOTE-061: global health status/quality of life scores over time with pembrolizumab and paclitaxel
KEYNOTE-062: first-line pembrolizumab monotherapy
Two different first-line pembrolizumab regimens were assessed by the global, randomized, placebo-controlled three-arm KEYNOTE-062 study that enrolled patients with advanced, PD-L1–positive (CPS ≥ 1) gastric or GEJ adenocarcinoma [17]. Pembrolizumab was administered either as monotherapy for up to 35 cycles (n = 256) or together with chemotherapy (n = 257). Patients in the control arm (n = 250) received placebo plus chemotherapy. CPS scores ≥ 10 prevailed in 79 %, 65 %, and 53 %, respectively. Microsatellite instability-high (MSI-H) status was found in 5 %, 7 %, and 8 %, respectively. More than two thirds of patients had been diagnosed with gastric cancer. OS and PFS were defined as coprimary endpoints.
Pembrolizumab monotherapy did not increase median OS compared to chemotherapy in the overall population (HR, 0.91), but reduced the mortality risk by 31 % in patients who had CPS ≥ 10 (17.4 vs. 10.8 months; HR, 0.69), although this was only an exploratory analysis. MSI-H expression enhanced OS benefits both in the total group (not reached vs. 8.5 months) and the CPS ≥ 10 cohort (not reached vs. 13.6 months). No PFS benefit was observed irrespective of PD-L1 expression. However, the pembrolizumab-treated patients in the MSI-H group did derive PFS improvement (11.2 vs. 6.6 months; HR, 0.72) as well as improved ORR (57.1 % vs. 36.8 %) and longer duration of response (21.2 vs. 7.0 months). In the overall population, response rates with pembrolizumab were relatively lower than in the control arm (CPS ≥ 1: 14.8 % vs. 37.2 %; CPS ≥ 10: 25.0 % vs. 37.8 %), although responses proved considerably more durable. Fewer patients in the pembrolizumab arm than in the chemotherapy arm experienced any-grade AEs.
Findings with pembrolizumab plus chemotherapy
With respect to the comparison between the pembrolizumab/chemotherapy arm and the chemotherapy-only arm, the additional benefit of the combination was generally modest. Pembrolizumab plus chemotherapy did not convey any OS improvement regardless of CPS scores. For PFS, only the results obtained for the CPS ≥ 10 cohort suggested some improvement (HR, 0.73). ORRs were relatively higher with the combination than with chemotherapy (CPS ≥ 1: 48.6 % vs. 37.2 %; CPS ≥ 10: 52.5 % vs. 37.8 %), with responses lasting longer in the CPS ≥ 10 population (8.3 vs. 6.8 months).
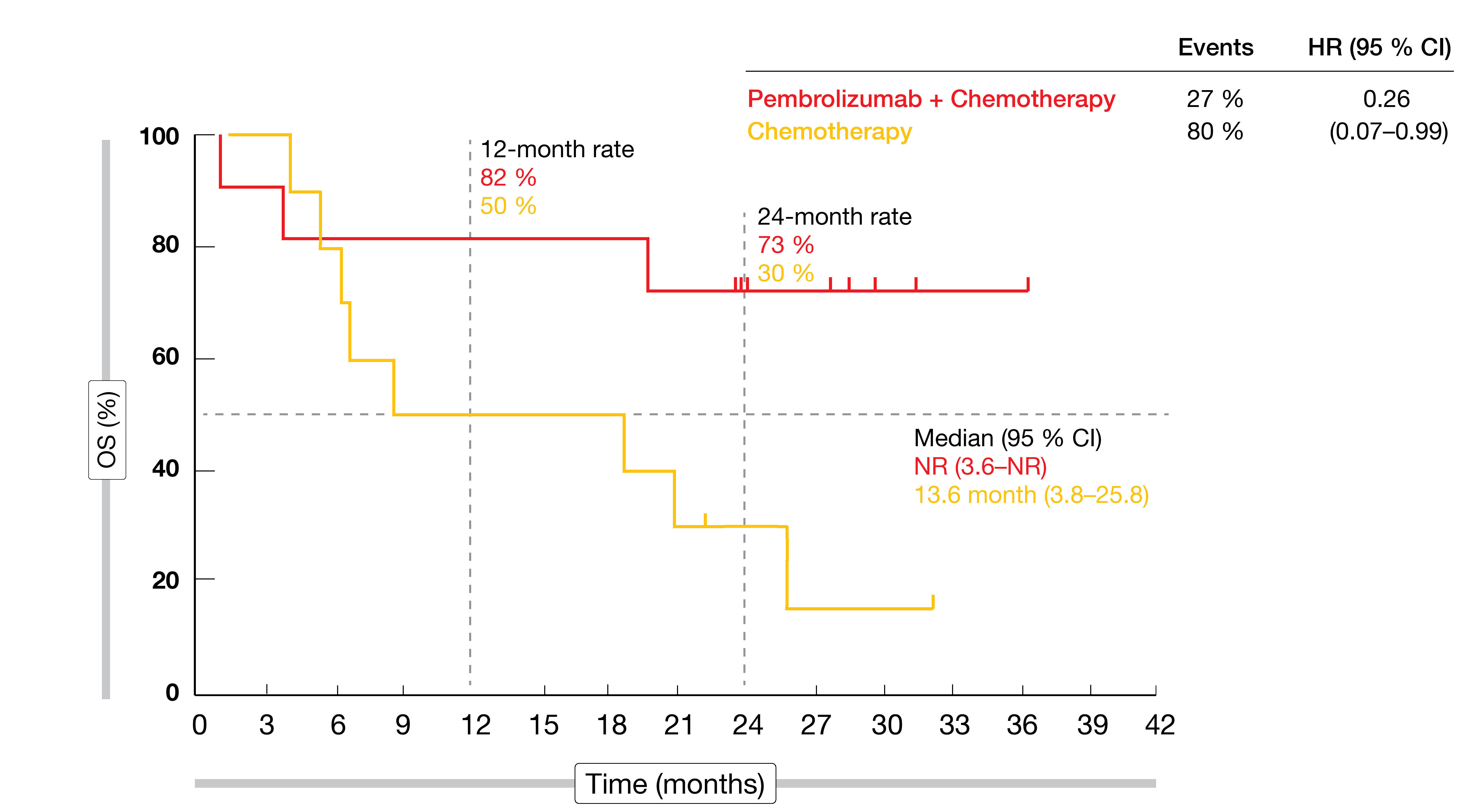
Efficacy outcomes were enhanced in the presence of MSI-H regardless of CPS status. MSI-H patients, when treated with both pembrolizumab and chemotherapy, derived substantial reductions in mortality risk compared to those receiving chemotherapy only (median OS in CPS ≥ 1: not reached vs. 8.5 months; HR, 0.37; CPS ≥ 10: not reached vs. 13.6 months; HR, 0.26; Figure 3). Also, substantial benefits resulted in the MSI-H population with regard to PFS (not reached vs. 6.6 months; HR, 0.45) and ORR (64.7 % vs. 36.8 %; duration of response, not reached vs. 7.0 months). Grade 3 to 5 AEs rates were similar across the two arms (73 % vs 69 %). Immune-related events occurred more often in the experimental arm but were mostly grade 1 or 2.
Figure 3: MSI-H cohort in the KEYNOTE-062 study: pembrolizumab plus chemotherapy vs. chemotherapy in patients with gastric or gastroesophageal carcinoma
Hepatocellular carcinoma
Liver cancer is the sixth most commonly diagnosed type of cancer worldwide, with hepatocellular carcinoma (HCC) representing 75 % to 85 % of cases [5]. Current systemic treatment options in the advanced stage include the tyrosine kinase inhibitors sorafenib and lenvatinib in the first line and regorafenib and cabozantinib in the second line, as well as the monoclonal antibody ramucirumab for patients with high serum alpha-fetoproteine levels. However, there is still an unmet need to prolong survival and improve tolerability. Research is focusing on the first- and second-line use of the PD-1 inhibitors pembrolizumab and nivolumab, as well as other aspects such as patient selection and quality of life.
CheckMate 459: nivolumab vs. sorafenib
The single-arm, phase I/II CheckMate 040 trial has yielded durable objective responses and promising long-term survival with nivolumab in advanced HCC with or without chronic viral hepatitis [18]. Based on these insights, nivolumab was approved by the FDA for the treatment of HCC in patients who have previously received sorafenib. The randomized CheckMate 459 study was designed to compare nivolumab with sorafenib in previously untreated patients with advanced HCC. Overall, 743 patients participated who had advanced HCC not amenable to surgical resection and/or loco-regional therapy (LRT) or had progressed after surgery and/or LRT. Positive PD-L1 tumor staining (> 1 %) was found in only approximately 20 %.
With respect to the primary outcome of OS, Checkmate 459 did not meet the predefined threshold of statistical significance, although OS improvement in the nivolumab arm was deemed clinically meaningful (16.4 vs. 14.7 months; HR, 0.85; p = 0.0752) [19]. Likewise, median PFS did not differ across arms (3.7 vs. 3.8 months; HR, 0.93), although at 24 months, a greater proportion of nivolumab-treated patients remained progression-free (14 % vs. 6 %). Objective responses occurred in 15 % versus 7 % (OR, 2.41), including a higher rate of complete remissions (4 % vs. 1 %).
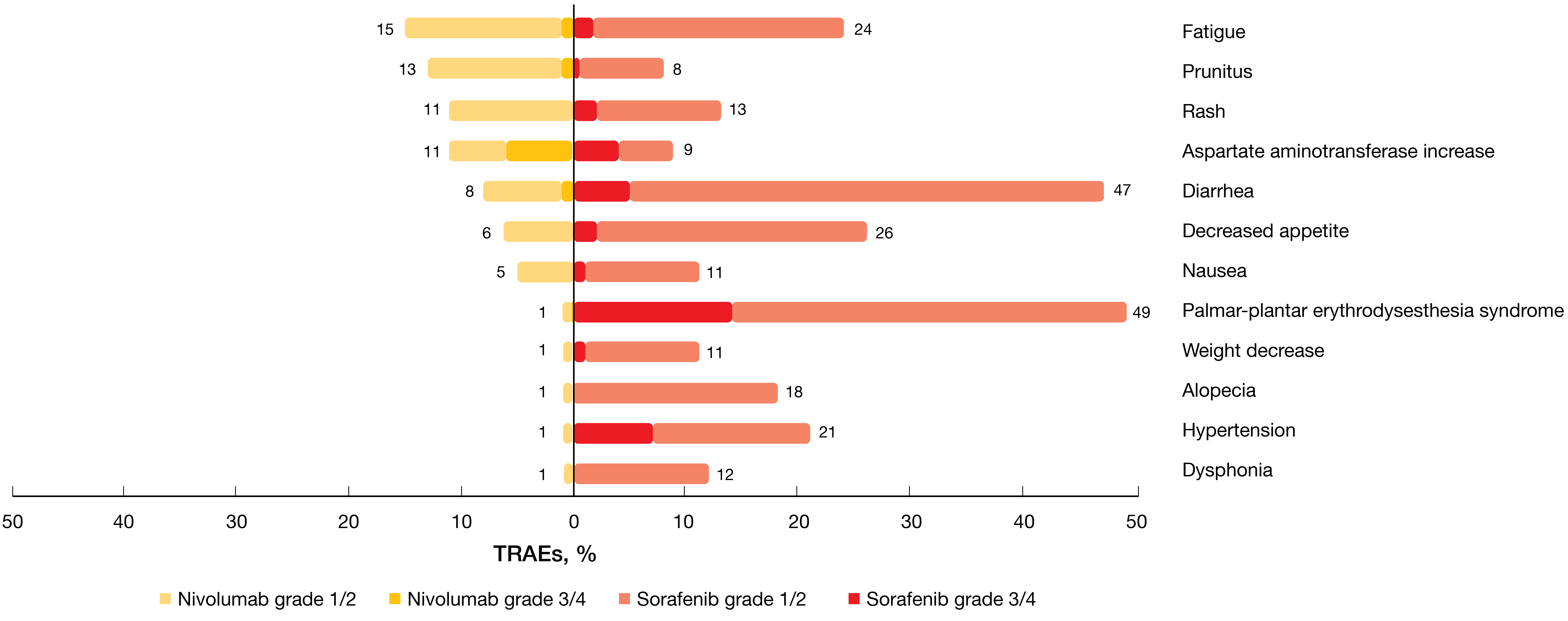
Overall survival results did not depend on PD-L1 baseline expression status, with a trend towards better OS in those with PD-L1 ≥ 1 % (HR, 0.80). Nivolumab demonstrated a favorable and manageable safety profile consistent with previous reports. Compared with sorafenib, fewer grade 3/4 TRAEs occurred (22 % vs. 49 %; Figure 4), which also applied to TRAEs leading to discontinuation. Also, the analysis demonstrated improved HRQoL according to the FACT-Hep questionnaire, with clinically meaningful differences in favor of nivolumab through week 113. Treatment burden was reduced in the experimental arm; through week 89, fewer patients who received nivolumab experienced worsening of side effects compared with sorafenib. Although CheckMate 459 did not meet its primary endpoint, the authors summarized that the study confirms the findings observed with second-line nivolumab in CheckMate 040.
Figure 4: Treatment-related adverse events observed in the CheckMate 459 study on first-line nivolumab vs. sorafenib in hepatocellular carcinoma
Real-world experience with nivolumab
In similar vein, real-world data collected with nivolumab at the Mount Sinai Hospital in New York resembled those obtained in the CheckMate 040 study [20]. One hundred and four patients with HCC received nivolumab, with 67 and 37 being treated in the first and subsequent lines, respectively. Among the later-line patients, 27 had progressed on sorafenib. In 31 %, LRT was performed concurrently. The median duration on treatment was 26 weeks, and median follow-up was 17 months.
Median OS was 23 and 12 months for patients treated in the first and subsequent lines, respectively. This difference did not reach statistical difference (p = 0.1013). Median PFS was estimated at 16 and 6 months, respectively. Ten percent of patients achieved complete remissions; all of these received LRT before or during the application of nivolumab. Partial responses and stable diseases were observed in 11 % and 38 %, respectively. In complete and partial responders, median OS had not been reached yet at the time of the analysis, while it was 23 months in patients who obtained disease stabilization.
HRQoL in the KEYNOTE-240 trial
Like nivolumab, pembrolizumab has received accelerated FDA approval for the treatment of sorafenib-pretreated patients with HCC; this was based on the results of the open-label phase II KEYNOTE-224 study [21]. In the phase III setting, the double-blind, randomized, placebo-controlled KEYNOTE-240 trial tested best supportive care plus either pembrolizumab or placebo in 413 patients with advanced HCC who showed progression on or intolerance to sorafenib. OS and PFS indeed favored pembrolizumab here, although the results did not meet significance according to the pre-specified statistical plan [22].
At ESMO 2019, Merle et al. reported the pre-specified exploratory HRQoL analyses that were conducted in KEYNOTE-240 using the EORTC QLQ-C30 and EORTC QLQ-HCC18 questionnaires [23]. In light of the poor patient prognosis, the impact of treatment on quality of life is an important consideration in HCC. The HRQoL population included 398 patients, with 271 and 127 randomly assigned to pembrolizumab and placebo, respectively.
The EORTC QLQ-C30 global health status/quality of life scores remained stable in both treatment groups. At 12 weeks, EORTC QLC-C30 and EORTC QLQ-HCC18 scores as well as all functional and symptoms domain scores were similar between pembrolizumab and placebo. Time to deterioration did not differ for the pre-specified symptoms of abdominal swelling, fatigue, and pain according to EORTC QLQ-HCC18. The authors concluded that these data, together with the efficacy and safety results from KEYNOTE-240, indicate a favorable risk-benefit balance for pembrolizumab in the second-line setting.
Lenvatinib plus pembrolizumab
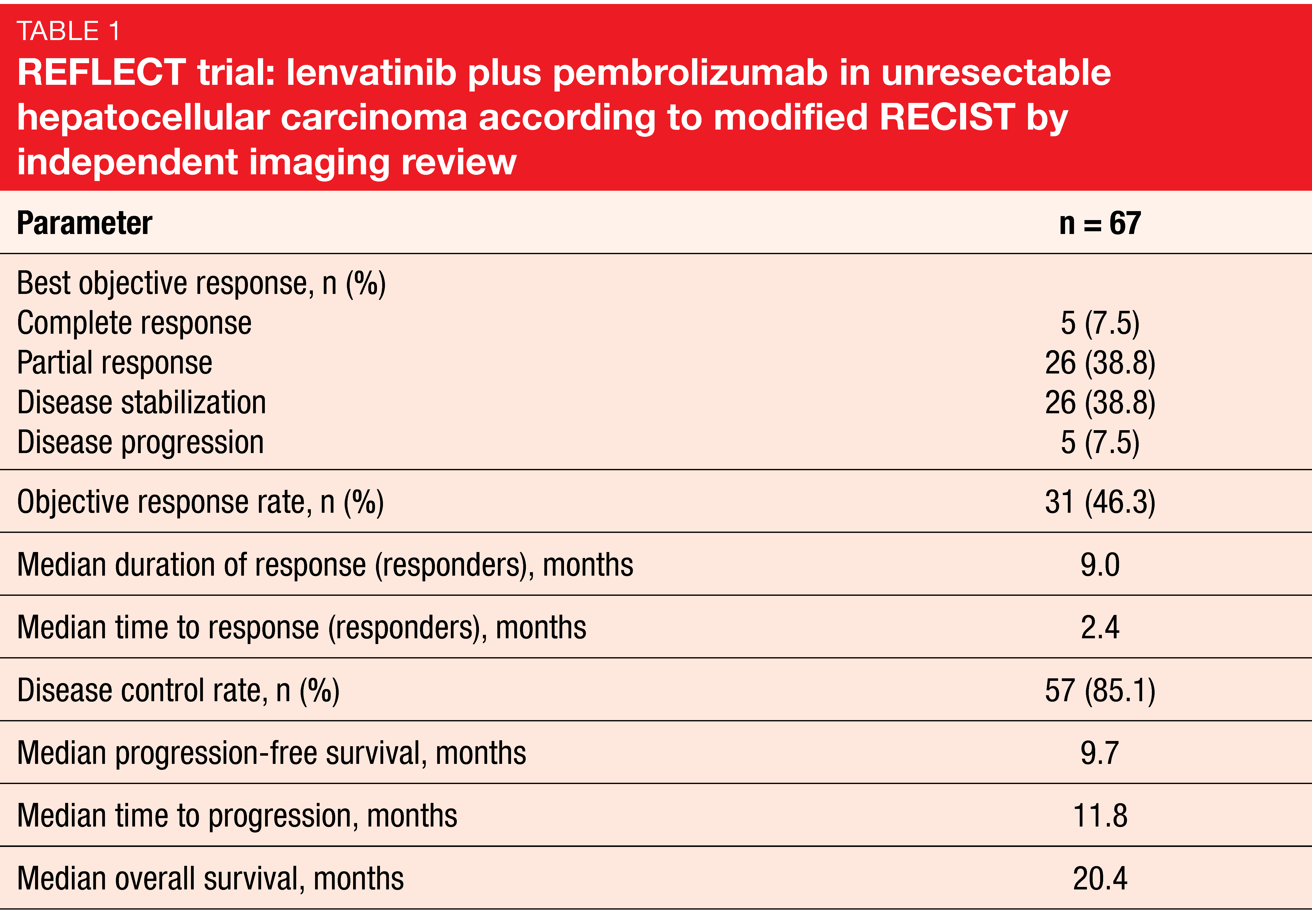
The anti-angiogenic multikinase inhibitor lenvatinib has been approved for the first-line treatment of unresectable HCC in many countries worldwide based on the findings obtained in the phase III REFLECT study [24]. Given potential synergistic effects between lenvatinib and pembrolizumab, an open-label, phase Ib study assessed lenvatinib 12 or 8 mg/d (depending on body weight) plus pembrolizumab in patients with unresectable HCC. After part 1 (n = 6) had revealed no dose-limiting toxicities, the protocol was amended to enroll approximately 94 patients to the part 2 expansion cohort. Sixty-seven patients were included in the follow-up analysis presented at ESMO 2019, with almost half of them still undergoing study treatment at the time of data cutoff [25].
Indeed, the results demonstrated strong anti-tumor activity of lenvatinib plus pembrolizumab, with a 46.3 % confirmed ORR by modified RECIST and independent imaging review (Table 1) and median PFS of 9.7 months. Disease control occurred in 85.1 %. Most patients experienced reductions in tumor size that appeared to be durable. Median OS amounted to 20.4 months. No unexpected safety signals were observed, and toxicities were manageable with dose modifications and interruptions. The combination of lenvatinib and pembrolizumab has been granted a Breakthrough Therapy designation for patients with advanced unresectable HCC who are not amenable to locoregional treatment.
Novel PD-1 inhibition: tislelizumab
The investigational humanized IgG4 monoclonal antibody tislelizumab was engineered to minimize binding to FcγR on macrophages in order to abrogate antibody-dependent phagocytosis, a mechanism of T-cell clearance and potential resistance to anti-PD-1 therapy. Tislelizumab shows higher affinity for PD-1 than pembrolizumab and nivolumab, with an approximately 100- and 50-fold slower off-rate, respectively [26]. The novel PD-1 inhibitor is currently being tested in clinical studies at a dose of 200 mg every 3 weeks. Analyses presented at ESMO 2019 aimed to determine the feasibility of extended dosing schedules and to develop a population pharmacokinetic (PK) model for tislelizumab.
Data supporting the 6-week regimen
Extended dosing schedules have already proven feasible in other PD-1 inhibitors such as nivolumab and pembrolizumab, which can be administered every 4 or 6 weeks, respectively. Wu et al. conducted an exposure-response analysis for tislelizumab in subjects with advanced tumors to inform the benefit-risk assessment and to explore the feasibility of alternative schedules [27]. The relationships between tislelizumab exposure and both efficacy and safety endpoints were tested using data collected from the three clinical BGB-A317-001, BGB-A317-102, and BGB-A317-203 studies. These trials included a total of 745 patients with solid tumors (e.g., gastric/esophageal cancer, HCC, ovarian cancer, non-small-cell lung cancer, urothelial carcinoma) and 70 patients with classical Hodgkin’s lymphoma. The individual model-predicted PK parameters used as the exposure measures comprised steady-state trough and peak concentrations, as well as time-averaged concentrations over the first 42 days and at steady-state. For response measures, ORR was defined as the efficacy endpoint. Safety endpoints included immune-related AEs, infusion-related AEs, grade > 3 AEs, AEs leading to dose modification, and those leading to drug discontinuation.
This analysis showed a lack of clinically significant exposure-response relationships for ORR and safety endpoints across the range of tested solid tumors and Hodgkin lymphoma, which supports the evaluation of the 6-weekly 400 mg regimen in future clinical trials. This regimen is not expected to be clinically different from the 3-weekly 200 mg schedule in terms of safety and efficacy outcomes.
Linear pharmacokinetics
Another analysis based on the BGB-A317-001, BGB-A317-102, and BGB-A317-203 studies was conducted to develop a population PK model for tislelizumab and to quantify the impact of demographic and disease characteristics on tislelizumab pharmacokinetics [28]. Typical values and interpatient variability of PK parameters in cancer patients were estimated, and the effects of demographic, pathophysiologic, and disease-related covariates on the PK of tislelizumab were determined to better understand clinical factors that might affect exposure in individual patients. The final population PK model was developed from a dataset of 798 subjects.
Tislelizumab PK was confirmed to be linear in the dose range tested. The authors noted that it can be adequately described by a three-compartment disposition model with linear clearance. No time-varying clearance was observed in this analysis. The covariates tested did not have a clinically meaningful impact on tislelizumab exposure.
Sensitivity analysis results support the use of the current clinical dose of 200 mg every 3 weeks. No dose adjustment appeared necessary based on patient age, body weight, race, sex, tumor type, and tumor size.
Urothelial carcinoma
Urothelial carcinoma is the most common type of bladder cancer. Until recently, initial treatment options for patients with metastatic urothelial carcinoma were limited to platinum-based chemotherapy regimens. However, a considerable proportion of patients with advanced disease cannot receive standard chemotherapy because of renal dysfunction, poor performance status, or other comorbidities. Therefore, the availability of additional options is a highly unmet medical need.
PD-1 inhibitors offer new possibilities here. Pembrolizumab has shown activity in both advanced urothelial carcinoma and non–muscle-invasive bladder cancer unresponsive to Bacillus Calmette-Guérin. Phase II data generated for the novel agent tislelizumab in a Chinese population denote it as a promising agent in urothelial carcinoma.
KEYNOTE-045: later-line pembrolizumab
Pembrolizumab has received approval for the second-line treatment of locally advanced or metastatic platinum-refractory urothelial carcinoma based on the phase III KEYNOTE-045 study. In this trial, pembrolizumab gave rise to survival prolongation and improved tolerability compared with paclitaxel, docetaxel, or vinflunine as per investigator’s choice (10.3 vs. 7.4 months) [29]. Patients with urothelial carcinoma of the renal pelvis, ureter, bladder, or urethra who had disease progression after one or two lines of platinum-based chemotherapy or recurrence within 12 months after perioperative platinum-based therapy were randomized to either pembrolizumab (n = 270) or chemotherapy (n = 272). Even after more than 2 years, the PD-1 inhibitor showed durable clinical benefit.
At ESMO 2019, Necchi et al. presented the 3-year follow-up from the KEYNOTE-045 study [30]. According to this analysis, pembrolizumab continued to show substantial improvement compared with chemotherapy. Median OS was 10.1 vs. 7.2 months (p = 0.00030). At 36 months, 20.7 % vs. 11.0 % of patients were alive. OS benefits with pembrolizumab were observed across subgroups.
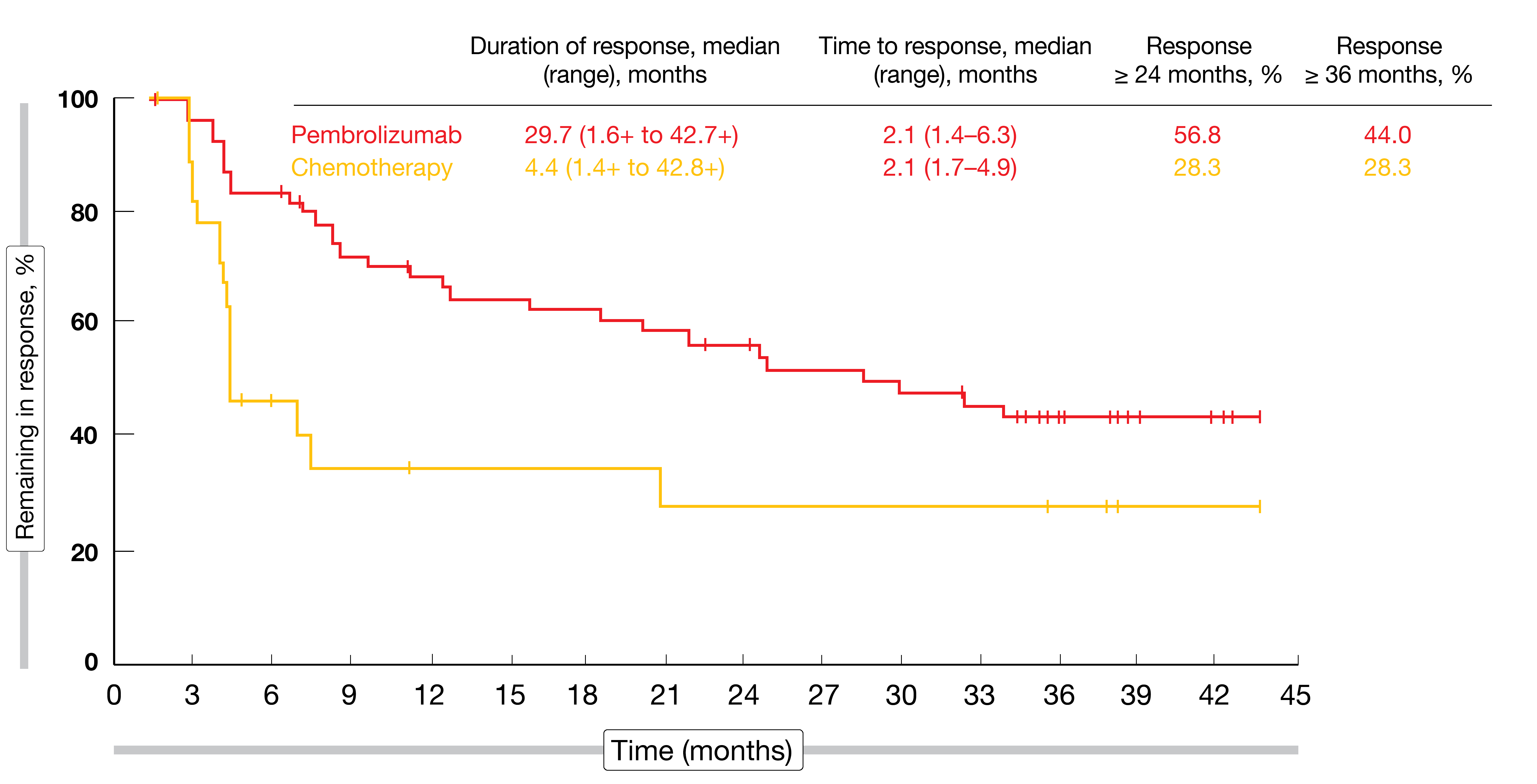
Although median PFS was not improved in the experimental arm (HR, 0.96), the 36-month PFS rate favored pembrolizumab (9.8 % vs. 2.0 %), suggesting a long-term PFS benefit for some patients. At 36 months, 22 patients (10 %) in the pembrolizumab arm remained progression-free; in this group, complete and partial remissions occurred in 68.2 % and 27.3 %, respectively. In the overall cohort, ORRs were 21.1 % vs. 11.0 %, with complete responses observed in 9.6 % vs. 2.9 %. Among patients who achieved complete or partial remissions, duration of response was 29.7 vs. 4.4 months, and responses that lasted for ≥ 36 months were found in 44.0 % vs. 28.3 % (Figure 5). The safety profile of pembrolizumab was better than that of chemotherapy, with lower rates of TRAEs (62.0 % vs. 90.6 %) and grade 3 to 5 TRAEs (16.9 % vs. 50.2 %).
Figure 5: Duration of response and time to response in patients with complete or partial remissions on second-line pembrolizumab vs. chemotherapy in urothelial carcinoma
Non–muscle-invasive tumors: KEYNOTE-057
Approximately 75 % of patients with urothelial carcinoma of the bladder present with tumors confined to the mucosa and submucosa. In patients with high-risk non–muscle-invasive bladder cancer (HR NMIBC), standard-of-care therapy is transurethral resection and intravesical Bacillus Calmette-Guérin (BCG) [31], although responses are often not durable. Due to the high risk of disease progression, radical cystectomy is a recommended standard option for patients with BCG-unresponsive NMIBC. However, surgery results in significant morbidity and mortality and has a negative impact on quality of life. There is an unmet need for novel therapies to reduce the risk of recurrence and raise bladder preservation rates.
A rationale for the use of anti-PD-1 therapies exists here as the PD-1 pathway has been implicated in BCG resistance [32]. De Wit et al. hypothesized that the use of pembrolizumab will result in clinically meaningful and durable complete response rates in HR NMIBC that is unresponsive to BCG therapy. Therefore, the open-label, single-arm, multicenter, phase II KEYNOTE-057 study tested pembrolizumab in patients with HR NMIBC with carcinoma in situ with or without papillary tumors (cohort A) or patients who had HR NMIBC without carcinoma in situ (cohort B). At ESMO 2019, the updated results from cohort A including HRQoL findings were reported [33].
Striking complete response rates
Patients enrolled in this cohort (n = 102) had histologically confirmed carcinoma in situ with or without papillary disease of predominantly transitional cell histology, BCG-unresponsive disease despite adequate BCG therapy, and were ineligible for or refused to undergo radical cystectomy. Pembrolizumab continued to show encouraging anti-tumor activity with a compelling complete response rate of 41.2 %. Forty-five percent of the 42 complete responders had ongoing responses at the time of data cutoff, while 47.6 % experienced recurrent NMIBC after complete remission. No patient developed muscle-invasive or metastatic disease while on study therapy. Median duration of CR was 16.2 months, and 41.3 % of patients responded for at least 18 months. Median OS had not been reached yet, which also applied to median PFS to worsening of grade/stage or death and PFS to muscle-invasive or metastatic disease or death. For the two PFS endpoints, 12-month rates were 83.4 % and 96.9 %, respectively. Ninety-eight percent of patients were alive at 12 months. Among patients who never achieved complete remission (n = 60), 46.7 % underwent cystectomy after a median time of 2.6 months from the last pembrolizumab dose. In the group of initial complete responders whose disease recurred (n = 23), 43.5 % had cystectomy after discontinuation; here, the median time from the last pembrolizumab dose to surgery was 4.2 months.
Pembrolizumab showed an AE profile consistent with observations from previous studies. HRQoL was measured by the Functional Assessment of Cancer Therapy-Bladder Cancer (FACT-BI) and Core Lower Urinary Tract Symptom Score (CLSS). Both patient-reported outcome instruments showed that HRQoL was maintained among pembrolizumab-treated patients. HRQoL and symptom scores were stable from baseline to week 51. According to a pre-specified analysis conducted in week 39, cancer-specific subscales and physical well-being scores from baseline were improved or stable in more than 70 % of patients. The phase III KEYNOTE-676 study is currently evaluating pembrolizumab plus BCG in patients with HR NMIBC that persists or has recurred after BCG induction.
Phase II data demonstrate activity of tislelizumab
Urothelial carcinoma is one of the most common urological malignancies in China. According to cancer statistics for China, bladder cancer accounted for approximately 80,500 new cancer cases and 32,900 deaths in 2015 [34]. The novel PD-1 inhibitor tislelizumab might contribute to expanding the immunotherapeutic armamentarium in this patient group. Recent data from two phase I studies (NCT02407990; CTR20160872) conducted in patients with urothelial carcinoma suggested that single‑agent tislelizumab was generally well tolerated and demonstrated antitumor activity (data on file). Clinical responses were observed for both PD-L1–positive and PD-L1–negative/unknown tumors, with ORRs amounting to 24 % and 21 %, respectively.
The single-arm, multicenter, phase II CTR20170071 study was conducted in China and other Asian countries to evaluate tislelizumab at the recommended phase II dose of 200 mg every 3 weeks in patients with locally advanced or metastatic PD-L1–positive urothelial carcinoma previously treated with ≥ 1 platinum-containing therapy [35]. PD-L1 positivity was present if ≥ 25 % of tumor cells or immune cells had PD-L1 expression. ORR was defined as the primary endpoint. A total of 113 patients received treatment. Overall, 104 patients were evaluable for tumor response.
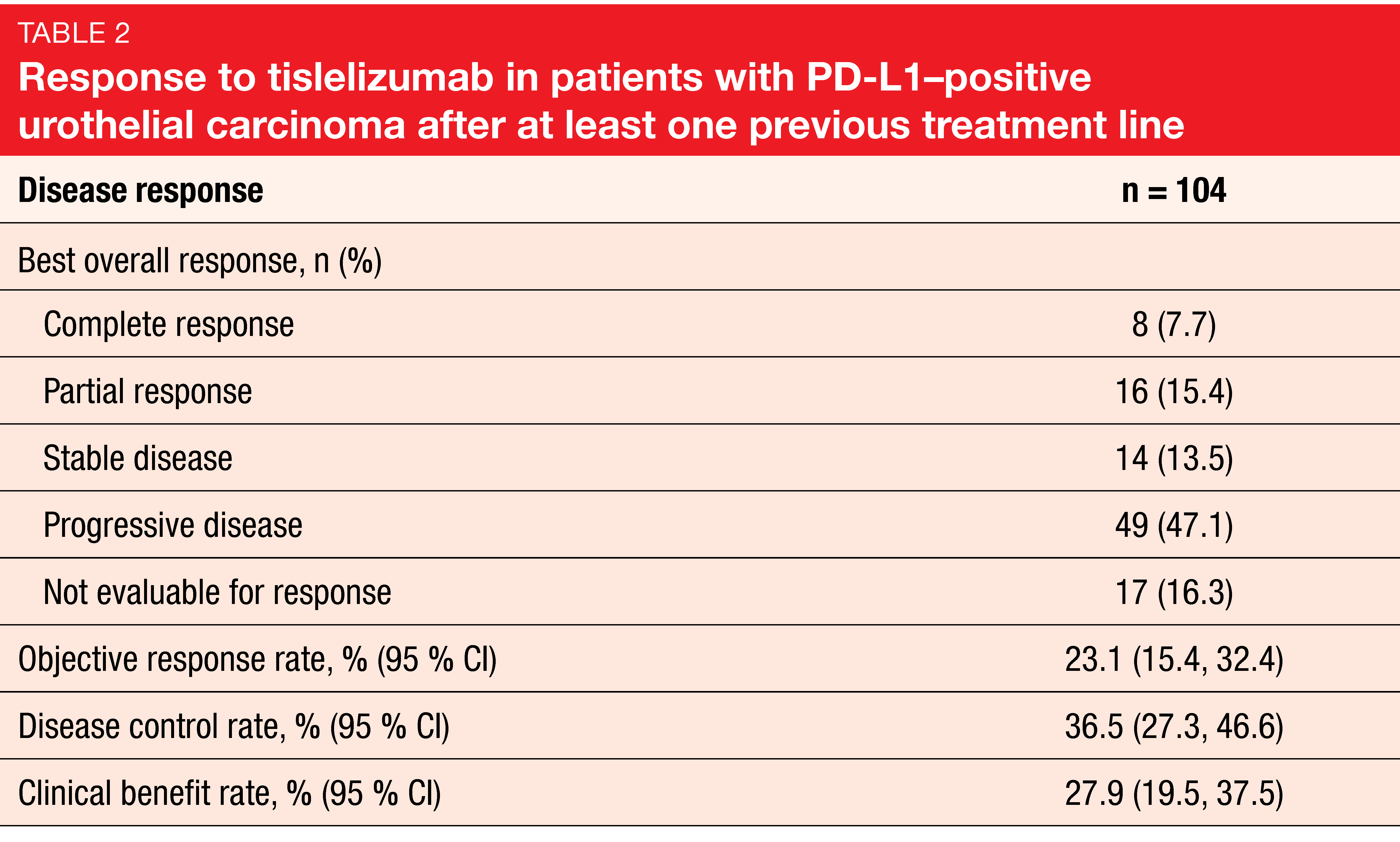
Disease control in a third of patients
At cutoff, median study follow-up was 7.6 months, and 30 patients remained on treatment. The median duration of treatment was 15.3 weeks. Confirmed objective responses occurred in 24 patients (23.1 %), including 8 complete and 16 partial responses according to independent review committee assessment (Table 2). Disease control and clinical benefit rates amounted to 36.5 % and 27.9 %, respectively. The subgroup analyses indicated that response rates were not considerably influenced by baseline factors. As the authors noted, the response rates reported here were similar to pooled data from the two phase I studies mentioned above that investigated tislelizumab in PD-L1–positive and PD-L1–negative/unknown tumors.
Thirty-four patients (33%) had reductions of ≥ 30 % in the sum of target lesion diameter from baseline. At the data cutoff date, the median duration of response had not been reached yet, with 79 % of responders showing ongoing responses. Median PFS and OS were 2.1 and 9.8 months, respectively. At 12 months, 46.5 % and 16.8 % of patients were alive and progression-free, respectively. Tislelizumab was generally well tolerated. The only treatment-emergent AEs occurring in > 15 % of patients included anemia (27 %), decreased appetite (19 %), and pyrexia (17 %). Anemia (7 %) was the only grade 3-4 treatment-emergent AE reported in ≥ 5 % patients. A total of 64 % of patients experienced immune-related treatment-emergent AEs, although no grade ≥ 3 immune-related events occurred in > 5 % of patients. Based on preliminary results from this trial, tislelizumab has received a priority review by China’s National Medical Product Administration.
REFERENCES
- World Cancer Research Fund. http://www.wcrf.org/int/cancer-facts-figures/worldwide-data (accessed August 2018)
- Torre LA et al., Global cancer statistics, 2012. CA Cancer J Clin 2015; 65(2): 87-108
- GLOBOCAN Stomach Cancer Fact Sheet. http://globocan.iarc.fr/old/FactSheets/cancers/stomach-new.asp (accessed August 2018)
- Pasechnikov V et al., Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol 2014; 20(38): 13842-13862
- Bray F et al., Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin 2018; 68(6): 394-424
- International Agency for Research on Cancer. Cancer Today 2018. https://gco.iarc.fr/today (accessed September 12, 2019)
- Lin Y et al., Epidemiology of esophageal cancer in Japan and China. J Epidemiol 2013; 23(4): 233-242
- https://cancerstatisticscenter.cancer.org/#!/ (accessed September 9, 2019)
- https://ganjoho.jp/en/profressional/statistics/table_download.html (accessed September 9, 2019)
- Cho BC et al., Nivolumab versus chemotherapy in advanced esophageal squamous cell carcinoma: the ATTRACTION-3 study. ESMO 2019, abstract LBA11
- Kang YK et al., Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 201; 390(10111): 2461-2471
- Sunakawa Y et al., Interim analysis of an observational/translational study for nivolumab treatment in advanced gastric cancer: JACCRO GC-08 (DELIVER trial). ESMO 2019, abstract 820P
- Kojima T et al., Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: Phase III KEYNOTE-181 study. J Clin Oncol 37, 2019 (suppl 4; abstr 2)
- Chen J et al., Pembrolizumab versus chemotherapy in patients with advanced/metastatic adenocarcinoma of squamous cell carcinoma of the esophagus as second-line therapy, analysis of the Chinese subgroup in KEYNOTE-181. ESMO 2019, abstract 760P
- Wainberg Z et al., KEYNOTE-059 update: efficacy and safety of pembrolizumab alone or in combination with chemotherapy in patients with advanced gastric or gastroesophageal (G/GEJ) cancer. ESMO 2017, abstract LBA28_PR
- Van Cutsem E et al., Impact of pembrolizumab versus paclitaxel on HRQoL in patients with advanced gastric or gastroesophageal junction cancer that has progressed after first-line chemotherapy (KEYNOTE-061). ESMO 2019, abstract 791P
- Shitara K et al., Pembrolizumab with or without chemotherapy vs chemotherapy in patients with advanced G/GEJ cancer including outcomes according to microsatellite instability-high status in KEYNOTE-062. ESMO 2019, abstract LBA44
- El-Khoueiry AB et al., Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389(10088): 2492-2502
- Yau T et al., CheckMate 459: a randomized, multi-center phase 3 study of nivolumab vs sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma. ESMO 2019, LBA38
- Dharmapuri S et al., Outcomes of hepatocellulcar carcinoma patients treated with nivolumab: the Mount Sinai Hospital (New York) experience. ESMO 2019, abstract 759P
- Zhu AX et al., Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018; 19(7): 940-952
- Finn RS et al., Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol 37, 2019 (suppl; abstr 4004)
- Merle P et al., Health-related quality-of-life impact of pembrolizumab versus best supportive care in previously systemically treated patients with advanced hepatocellular carcinoma: KEYNOTE-240. ESMO 2019, abstract 676PD
- Kudo M et al., Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018; 391(10126): 1163-1173
- LLovet JM et al., A phase Ib trial of lenvatinib plus pembrolizumab in unresectable hepatocellular carcinoma: updated results. ESMO 2019, abstract 747P
- Feng Y et al., The molecular binding mechanism of tislelizumab, an investigational anti-PD-1 antibody, is differentiated from pembrolizumab and nivolumab. 110th Annual Meeting of the American Association for Cancer Research, abstract 2382
- Wu CY et al., Tislelizumab exposure-response analyses of efficacy and safety in patients with advanced tumors. ESMO 2019, abstract 482P
- Wu CY et al., Population pharmacokinetics of tislelizumab in patients with advanced tumors. ESMO 2019, abstract 483P
- Fradet Y et al., Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of > 2 years of follow-up. Ann Oncol 2019; May 3. pii: mdz127. doi: 10.1093/annonc/mdz127. [Epub ahead of print]
- Necchi A et al., Three-year follow-up from the phase 3 KEYNOTE-045 trial: pembrolizumab versus investigator’s choice (paclitaxel, docetaxel, or vinflunine) in recurrent, advanced urothelial cancer. ESMO 2019, abstract 919P
- NCCN Clinical Practice Guidelines (NCCN guidelines): bladder cancer (Version 4.2019)
- Fukumoto K et al., Clinical role of programmed cell death-1 expression in patients with non-muscle-invasive bladder cancer recurring after initial Bacillus Calmette-Guérin therapy. Ann Surg Oncol 2018; 25(8): 2484-2491
- De Wit R et al., Health-related quality of life and updated follow-up from KEYNOTE-057: phase 2 study of pembrolizumab for patients with high-risk non–muscle-invasive bladder cancer unresponsive to Bacillus Calmette-Guérin. ESMO 2019, abstract 916P
- Chen W et al., Cancer statistics in china, 2015. CA Cancer J Clin 2016; 66(2): 115-132
- Dingwei Y et al., First report of efficacy and safety from a phase 2 trial of tislelizumab, an anti-PD-1 antibody, for the treatment of PD-L1+ locally advanced or metastatic urothelial carcinoma in Asian patients. ESMO 2019, abstract 920P