Milestones of PD-1 inhibition in gastric and esophageal cancer
Gastric cancer, gastroesophageal junction (GEJ) adenocarcinoma, and esophageal adenocarcinoma are substantial causes of cancer-related mortality worldwide and have poor 5-year overall survival (OS) when diagnosed at an advanced stage [1, 2]. Median OS with standard first-line chemotherapy for advanced or metastatic, HER2-negative gastric and GEJ cancer is less than 1 year [3-6].
Several clinical trials investigating anti-PD-(L)1 monotherapy for gastric and GEJ cancer have yielded negative results. However, in 2017, nivolumab was shown to improve survival in patients with gastric and GEJ cancer included in the randomized, double-blind, placebo-controlled, phase III ATTRACTION-2 trial after at least two previous treatment lines [7]. The non-randomized phase II KEYNOTE-059 study demonstrated activity of pembrolizumab in the same setting [8]. Pembrolizumab monotherapy also proved beneficial in pretreated patients with advanced/metastatic adenocarcinoma or squamous cell carcinoma of the esophagus in the KEYNOTE-180 [9, 10] and KEYNOTE-181 [11] trials. Based on these studies, pembrolizumab was approved in the setting of recurrent, locally advanced or metastatic esophageal squamous cell carcinoma with combined positive score (CPS) ≥ 10 after ≥ 1 treatment line by the US Food and Drug Administration in 2019 [12]. Large phase III studies evaluating the benefits of nivolumab or pembrolizumab in combination with first-line chemotherapy for advanced gastric cancer, GEJ cancer and esophageal cancer were presented at ESMO 2020.
Phase III part of ATTRACTION-4
The randomized, multicenter, phase II/III ATTRACTION-4 study assessed nivolumab plus chemotherapy as first-line treatment in patients with HER2-negative, advanced gastric or gastroesophageal junction cancer. After the phase II part of the trial had shown encouraging results [13], Boku et al. reported the primary findings of the double-blind, randomized, controlled phase III part of ATTRACTION-4 at the ESMO 2020 Congress [14]. At 130 centers in Japan, Korean and Taiwan, patients received S-1 plus oxaliplatin or capecitabine plus oxaliplatin with either nivolumab 360 mg every 3 weeks (Q3W) or placebo until progression. Each arm contained 362 patients. Progression-free survival (PFS) and OS were defined as the coprimary endpoints.
For PFS, the nivolumab-based therapy proved superior compared to placebo plus chemotherapy, with a median of 10.45 vs. 8.34 months (HR, 0.68; p = 0.0007). At 1 year, 45.4 % vs. 30.6 % of patients were alive and progression-free. All of the subgroups benefited from the addition of the immune checkpoint inhibitor; this was also true regardless of PD-L1 expression (≥ 1 % vs. < 1 %). However, OS did not improve in a significant manner according to the final analysis. Median OS was 17.45 vs. 17.15 months (HR, 0.90; p = 0.257).
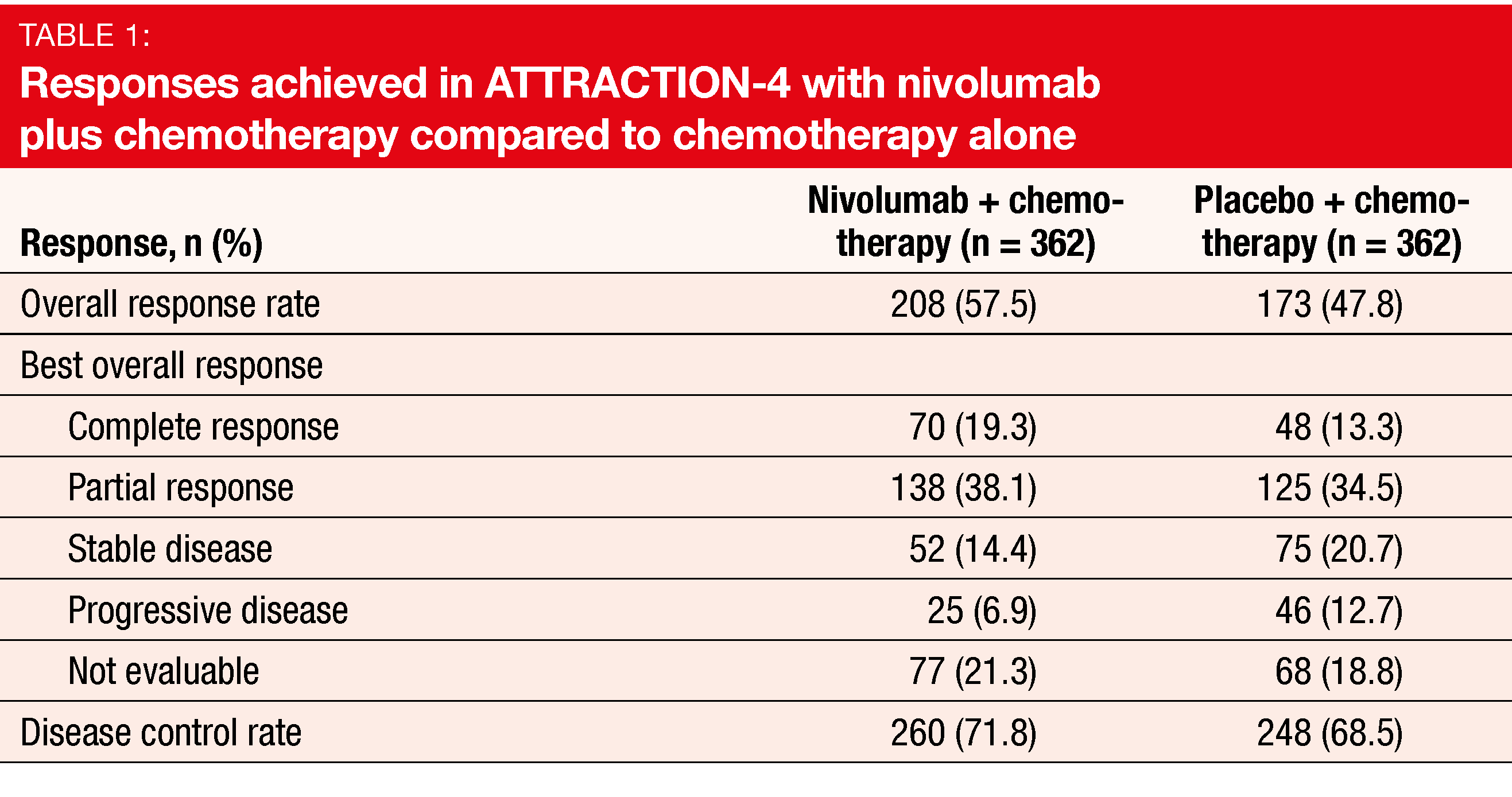
A greater proportion of patients treated in the experimental arm responded to the therapy (57.5 % vs. 47.8 %; p = 0.0088; Table 1). Duration of response was longer with nivolumab plus chemotherapy than with chemotherapy alone (12.91 vs. 8.67 months). The combination showed a manageable safety profile. AEs leading to discontinuation or dose delay/reductions occurred with comparable frequency across the two arms.
As the authors noted in their summary, the objective of the phase III part of ATTRACTION-4 was met, demonstrating clinically meaningful efficacy as per protocol the trial was to be deemed positive if at least one of the primary endpoints were met. Nivolumab plus chemotherapy could be considered a new first-line treatment option in patients with unresectable advanced or recurrent gastric or GEJ cancer.
CheckMate 649: insights based on almost 1,600 patients
The largest randomized, global phase III study investigating PD-1-inhibitor–based therapies in the first-line setting for patients with advanced gastric cancer, GEJ cancer, and esophageal adenocarcinoma is CheckMate 649. Möhler et al. reported the first results for the comparison between chemoimmunotherapy vs. chemotherapy at ESMO 2020 [15]. Approximately 790 patients with unresectable, advanced or metastatic HER2-negative tumors were randomized into each arm. Chemoimmunotherapy consisted of nivolumab 360 mg plus XELOX Q3W or nivolumab 240 mg plus FOLFOX Q2W, while the patients in the control arm received XELOX Q3W or FOLFOX Q2W alone.
OS testing was conducted hierarchically based on the observation that in gastric, GEJ and esophageal cancers, PD-L1 expression by CPS at a cutoff ≥ 5 shows better enrichment for the efficacy of checkpoint inhibitors than tumor cell PD-L1 expression [16]. The statistical plan specified that if OS in the PD-L1 CPS ≥ 5 population proved significantly superior, OS in the PD-L1 CPS ≥ 1 group was tested, followed by OS in all randomized patients. The PD-L1 CPS ≥ 5 population comprised 473 and 482 individuals in the experimental and control arms, respectively. For the PD-L1 CPS ≥ 1 group, this was 641 and 655, respectively. Results for the third arm of the CheckMate 649 study that assessed dual checkpoint inhibition with nivolumab plus ipilimumab followed by nivolumab monotherapy were not presented at this time.
Improvements in various groups
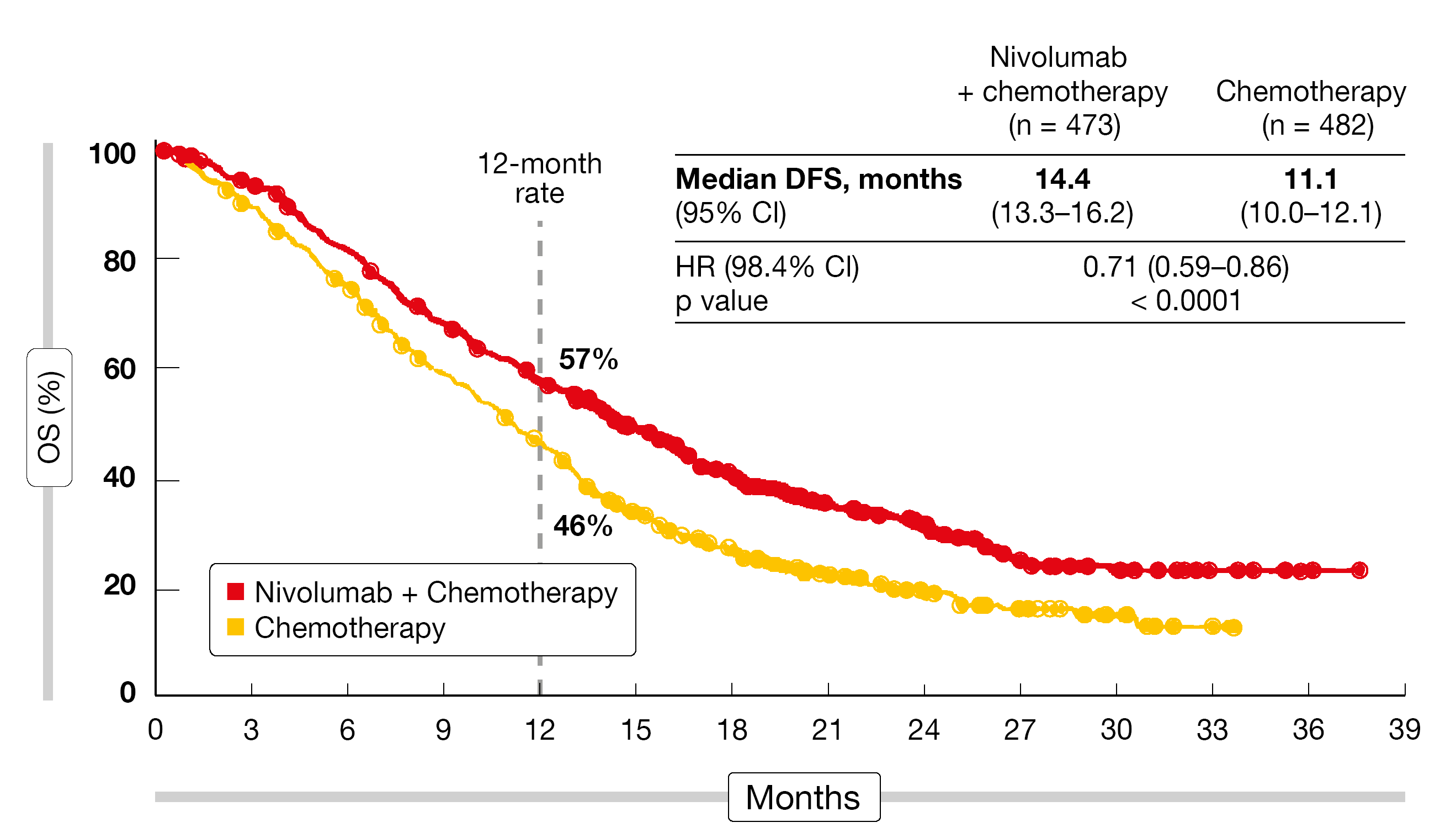
Overall survival and PFS in the CPS ≥ 5 population were defined as the dual primary endpoints. Indeed, the addition of nivolumab brought about a statistically significant and clinically relevant OS advantage in this group (14.4 vs. 11.1 months; HR, 0.71; p < 0,0001; Figure 1), as well as in the population with CPS ≥ 1 (14.0 vs. 11.3 months; HR, 0.77; p = 0.0001) and in all randomized patients (13.8 vs. 11.6 months; HR, 0.80; p = 0.0002). The OS findings consistently favored nivolumab plus chemotherapy across multiple subgroups. Likewise, PFS was significantly longer in the patients with CPS ≥ 5 who received nivolumab plus chemotherapy (7.7 vs. 6.0 months; HR, 0.68; p < 0.0001). Superiority of the experimental regimen with regard to PFS was also observed for the CPS ≥ 1 group (7.5 vs. 6.9 months; HR, 0.74) and the total randomized population (7.7 vs. 6.9 months; HR, 0.77). A significantly larger proportion of nivolumab-treated patients developed responses (60 % vs. 45 %; p < 0.0001), which were also more durable (9.5 vs. 7.0 months).
No new safety signals became apparent and the safety profile for the combination was consistent with the profiles of the individual agents. The most common any-grade treatment-related AEs (TRAEs) across both arms included nausea, diarrhea, and peripheral neuropathy. TRAEs occurred with similar incidence in the CPS ≥ 5 population and in all patients treated across both arms; this also applied to select TRAEs of potential immunologic etiology. Here, grade 3/4 events were seen in ≤ 5 % of patients. In their conclusion, the authors emphasized that nivolumab is the first PD-1 inhibitor to demonstrate superior OS and PFS in combination with chemotherapy compared to chemotherapy alone in previously untreated patients with advanced cancers of the stomach, GEJ and esophagus. The combination thus represents a new potential first-line standard in this setting.
Figure 1: Primary endpoint of CheckMate 649: overall survival benefit with nivolumab plus chemotherapy versus chemotherapy in the CPS ≥ 5 population
KEYNOTE-590: benefits of pembrolizumab
In patients with esophageal cancer, the randomized, double-blind, placebo-controlled, phase III KEYNOTE-590 study assessed pembrolizumab 200 mg Q3W for a maximum of 35 cycles plus 5-FU and cisplatin (n = 373) compared to placebo plus chemotherapy (n = 376) as first-line treatment [17]. The population included had locally advanced, unresectable or metastatic esophageal adenocarcinoma, squamous-cell carcinoma, or advanced/metastatic esophagogastric junction (EGJ) Siewert type 1 adenocarcinoma. Esophageal squamous-cell carcinoma (ESCC) was present in approximately 73 % in both arms. Among the patients with adenocarcinoma, roughly equal proportions had been diagnosed with esophageal or EGJ disease. Approximately half of all study participants in both arms showed PD-L1 CPS ≥ 10.
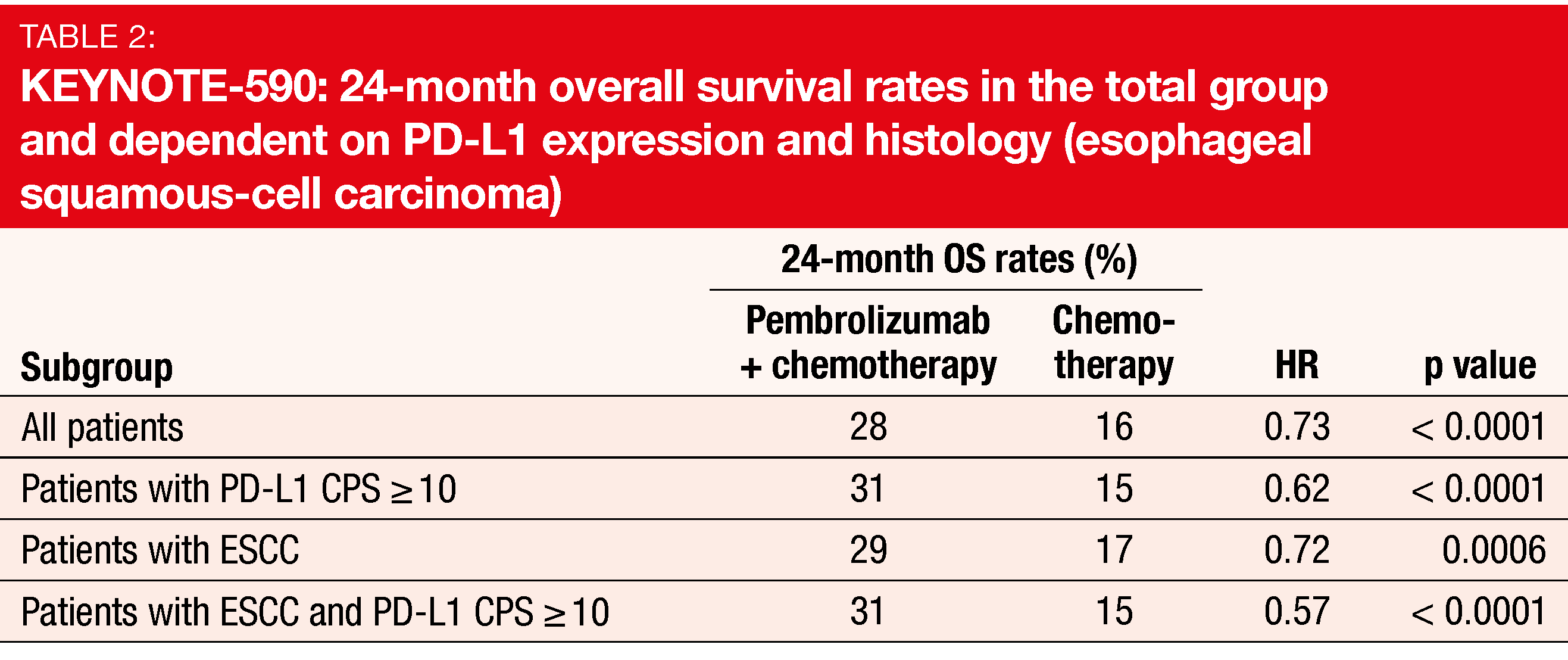
Indeed, first-line pembrolizumab plus chemotherapy provided statistically significant and clinically meaningful benefits in terms of several endpoints. Median OS was 12.4 vs. 9.8 months (HR, 0.73; p < 0.0001) in the total cohort, with a risk reduction of 27 %. Patients with PD-L1 CPS ≥ 10 experienced a 38 % reduction in their mortality risk (13.5 vs. 9.4 months; HR, 0.62; p < 0.0001). For the group with ESCC, OS was 12.6 vs. 9.8 months (HR, 0.72; p = 0.0006), and for those with ESCC and PD-L1 CPS ≥ 10, 13.9 vs. 8.8 months (HR, 0.57; p < 0.0001). The 24-month OS rates indicated sustained benefits in the experimental arm (Table 2). With respect to PFS, improvements were observed in the overall population (6.3 vs. 5.8 months; HR, 0.65; p < 0.0001), the ESCC cohort (6.3 vs. 5.8 months; HR, 0.65; p < 0.0001) and the PD-L1 CPS ≥ 10 group (7.5 vs. 5.5 months; HR, 0.51; p < 0.0001). All subgroups benefited from the addition of pembrolizumab with respect to OS and PFS. Within the entire cohort, 45.0 % of pembrolizumab-treated patients vs. 29.3 % of those in the control arm responded (p < 0.0001). Median duration of response amounted to 8.3 vs. 6.0 months.
Comparable safety profiles were reported for the two treatment groups. Grade ≥ 3 TRAEs were seen in 71.9 % vs. 67.6 % and necessitated treatment discontinuation in 19.5 % vs. 11.6 %. Immune-mediated AEs and infusion reactions occurred in 25.7 % vs. 11.6 %, with most events graded as mild or moderate. The authors concluded that pembrolizumab plus chemotherapy should be a new first-line standard of care in patients with locally advanced and metastatic esophageal cancer including EGJ adenocarcinoma.
Doubling of DFS with adjuvant nivolumab
In the setting of resectable locally advanced esophageal cancer and GEJ cancer, neoadjuvant chemoradiation therapy followed by surgery (i.e., trimodality therapy) is a widely used standard of care [18-20]. However, the risk of recurrence following trimodality therapy remains high, particularly in patients with residual pathologic disease, and established adjuvant treatment is lacking [18-21].
The adjuvant use of nivolumab was investigated in the CheckMate 577 trial, which is the first global, randomized, double-blind phase III study to evaluate a checkpoint inhibitor after trimodality therapy for esophageal/GEJ cancer [22]. At total of 794 patients with stage II/III disease and adenocarcinoma or squamous-cell histology were randomized to either nivolumab 240 mg Q2W for 16 weeks followed by 480 mg Q4W (n = 532) or placebo (n = 262). They had undergone neoadjuvant chemoradiotherapy and surgery within 4 to 16 weeks prior to randomization and had residual pathologic disease ≥ ypT1 or ≥ ypN1. The total treatment duration was up to 1 year.
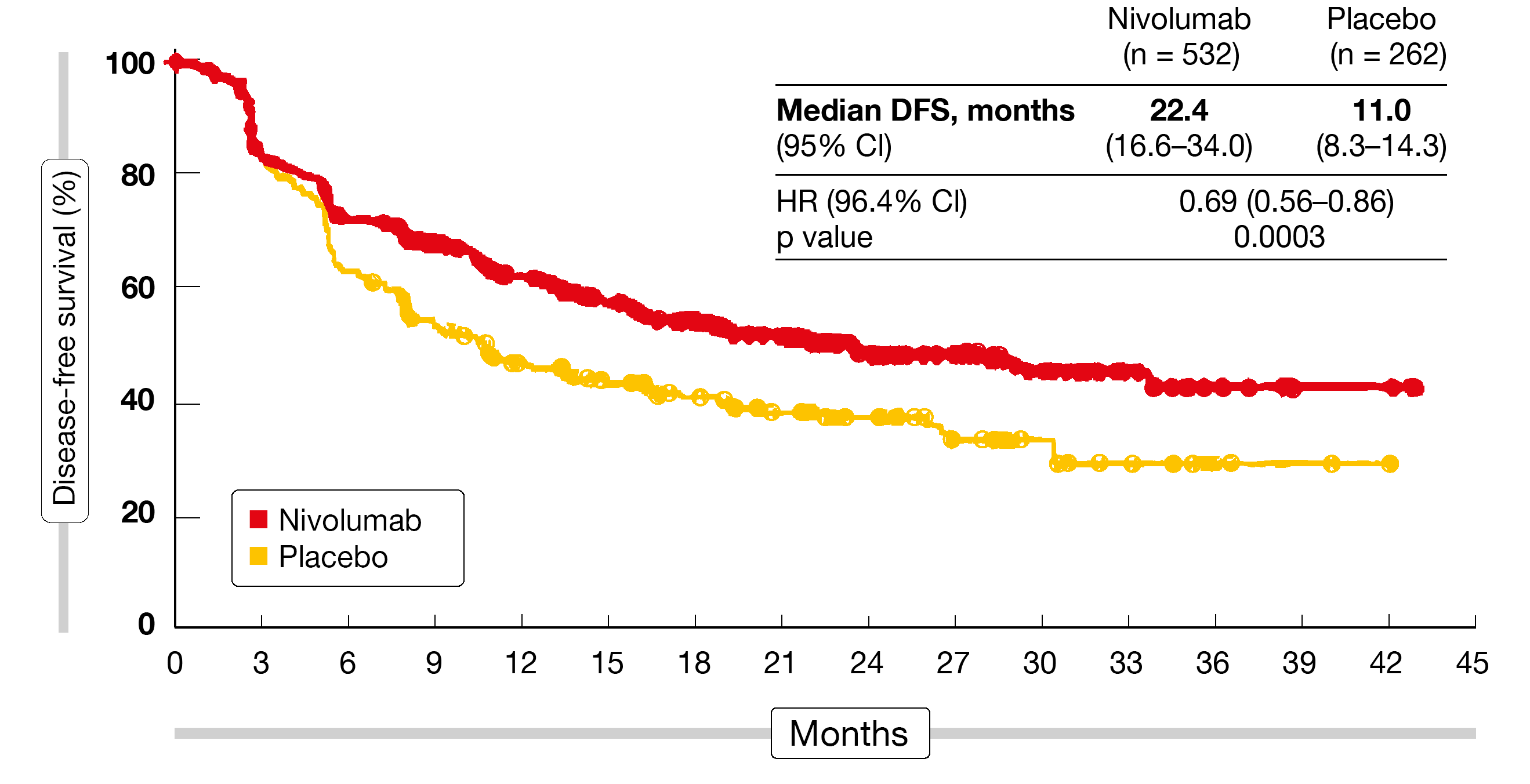
Disease-free survival constituted the primary outcome. Adjuvant nivolumab conferred a significant benefit here, with a 31 % reduction in the risk of recurrence or death (22.4 vs. 11.0 months; HR, 0.69; p = 0.0003; Figure 2). Findings in all predefined subgroups favored the PD-1 inhibitor over placebo. Nivolumab was well tolerated and showed an acceptable safety profile. The majority of TRAEs were grade 1 or 2, and only 9 % prompted treatment discontinuation. Fatigue, diarrhea, pruritus and rash were reported as the most common TRAEs. Grade 3/4 select TRAEs occurred in < 1 % of patients in the nivolumab arm. Correspondingly, patient-reported outcome analyses revealed similar overall health status with nivolumab and placebo according to the EQ-5D-3L instrument. As the authors noted, these results represent the first advance in years for patients with resected esophageal and GEJ cancer and potentially establish adjuvant nivolumab as a new standard of care.
Figure 2: Disease-free survival observed with adjuvant nivolumab in the CheckMate 577 trial
Visually estimated CPS vs. conventional CPS
Although approved PD-1 inhibitors have shown encouraging improvements in survival in patients with gastroesophageal adenocarcinoma, many patients do not respond, which highlights the need of predictive biomarkers. PD-L1 expression can be assessed using the CPS and the Dako 22C3 assay, although utilization of this scoring method can be challenging in clinical practice. Therefore, a less time-consuming algorithm based on visual estimation of the PD-L1 expression on tumor and immune cells named visually estimated CPS (vCPS) has been developed for the VENTANA PD-L1 (SP263) assay. Chao et al. compared the clinical utilization of CPS (with Dako 22C3) and vCPS (with VENTANA PD-L1 SP263) based on post-hoc analyses of samples from the first-in-human BGB-A317-001 study that tested the PD-1 inhibitor tislelizumab in patients with gastroesophageal adenocarcinoma [23]. In this group of 81 individuals, PD‑L1 expression was evaluable by CPS and vCPS in 49 and 74 patients with available formalin-fixed, paraffin-embedded tumors, respectively. Forty-five were evaluable using both assays.
The vCPS ≥ 5 % cutoff was determined as the optimal cutoff based on statistical analysis, prevalence, and pathological feasibility. This was further developed and analytically validated using the tumor samples. At the cutoffs assessed, both the VENTANA PD‑L1 (SP263) assay with vCPS ≥ 5 % and the commercialized Dako 22C3 assay with CPS ≥1 aided in the identification of patients with high PD-L1 expression who were more likely to benefit from treatment than those with PD-L1–low tumors. The reproducibility of the VENTANA PD‑L1 (SP263) assay with vCPS by different pathologists, materials, and laboratories indicated the highly trainable nature of the assay, as well as its consistency in gastric and GEJ adenocarcinoma.
Further clinical validation is underway for vCPS ≥ 5 % based on a phase III study designed to compare tislelizumab plus platinum/fluoropyrimidine versus placebo plus platinum/fluoropyrimidine as first-line therapy of gastric and GEJ cancer (RATIONALE 305; BGB-A317-305).
REFERENCES
- Ajani JA et al., Gastric adenocarcinoma. Nat Rev Dis Primers 2017; 3: 17036
- Rubenstein JH, Shaheen NJ. Epidemiology, diagnosis, and management of esophageal adenocarcinoma. Gastroenterology 2015; 149(2): 302-317
- Lordick F et al., Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013; 14 (6): 490-499
- Catenacci DVT et al., Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18(11): 1467-1482
- Shah MA et al., Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol 2017; 3(5): 620-627
- Fuchs CS et al., Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20(3): 420-435
- Kang YK et al., Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390(10111): 2461-2471
- Fuchs CS et al., Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 Trial. JAMA Oncol 2018; 4(5): e180013
- Shah et al., Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE-180 study. JAMA Oncol 2019; 5(4): 546-550
- Kato et al., Pembrolizumab in previously treated metastatic esophageal cancer: longer term follow-up from the phase 2 KEYNOTE-180 Study. J Clin Oncol 37, 2019 (suppl; abstr 4032)
- Shah et al., Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: Phase 3 KEYNOTE-181 study. J Clin Oncol 37, 2019 (suppl; abstr 4010)
- https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-advanced-esophageal-squamous-cell-cancer#:~:text=On%20July%2030%2C%202019%2C%20the,FDA%2Dapproved%20test%2C%20with%20disease
- Boku N et al., Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol 2019; 30(2): 250-258
- Boku N et al., Nivolumab plus chemotherapy versus chemotherapy alone in patients with previously untreated advanced or recurrent gastric/gastroesophageal junction cancer: ATTRACTION-4 (ONO-4538-37) study. ESMO 2020, LBA7_PR
- Möhler M et al., Nivolumab plus chemotherapy versus chemotherapy as first-line treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma: first results of the CheckMate 649 study. ESMO 2020, LBA6_PR
- Lei M et al., American Association for Cancer Research Annual Meeting 2019, abstract 2673
- Kato K et al., Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: the phase 3 KEYNOTE-590 Study. ESMO 2020, LBA8_PR
- NCCN Esophageal Cancer Guidelines V1.2020
- Lordick F et al., Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27(suppl 5): v50-v57
- Shah MA et al., Treatment of locally advanced esophageal carcinoma: ASCO Guideline. J Clin Oncol 2020 28(23): 2677-2694
- Blum Murphy M et al., Pathological complete response in patients with esophageal cancer after the trimodality approach: The association with baseline variables and survival-The University of Texas MD Anderson Cancer Center experience. Cancer 2017; 123(21): 4106-4113
- Kelly RJ et al., Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer following neoadjuvant chemoradiation therapy: first results of the CheckMate 577 study. ESMO 2020, LBA9
- Chao Y et al., Investigation of PD-L1 expression and tislelizumab efficacy in gastroesophageal adenocarcinoma using a novel tumor and immune cell score with VENTANA PD-L1 (SP263) assay and combined positive score (CPS). ESMO 2020, 154P
© 2020 Springer-Verlag GmbH, Impressum