Ovarian cancer: taking PARP inhibition one step further
Individualized dosing of niraparib
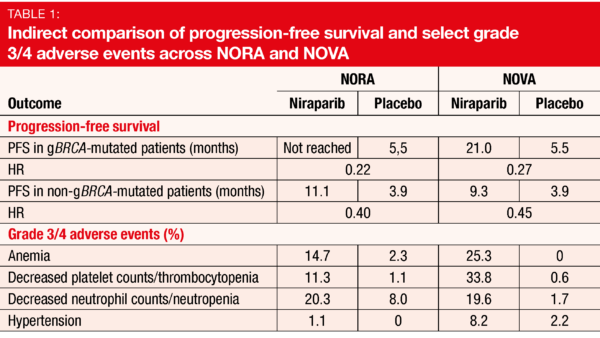
Niraparib has been approved as maintenance treatment for patients with platinum-sensitive recurrent ovarian cancer (OC) based on the results of the NOVA trial [1]. The starting dose used in NOVA was 300 mg orally daily. A retrospective analysis indicated that individualized starting doses based on baseline body weight and platelet counts might improve the safety profile of niraparib without compromising efficacy [2]. This approach was tested by the NORA study conducted in Chinese patients with platinum-sensitive, recurrent OC that was of high-grade serous or high-grade predominantly serous histology or germline BRCA-mutated [3]. The women had received at least 2 lines of platinum-containing therapy and had experienced partial or complete responses to the last treatment. They were randomized to either niraparib (n = 177) or placebo (n = 88) until disease progression. Eleven patients who had a baseline body weight ≥ 77 kg and platelet counts ≥ 150,000/µl received an initial niraparib dose of 300 mg daily, while 155 with < 77 kg and platelet counts < 150,000/µl were treated with 200 mg daily. PFS determined by blinded independent central review constituted the primary outcome. NORA is the first fully powered phase III, randomized, controlled study to evaluate a PARP inhibitor in Chinese patients with OC.
The trial met its primary endpoint. PFS was markedly prolonged in the experimental arm of the ITT population, with a 68 % reduction in the risk of mortality and progression (18.3 vs. 5.4 months; HR, 0.32; p < 0.0001). Both patients with and without germline BRCA mutations derived significant PFS benefits (p < 0.0001 each; Table 1). Also, patients in the niraparib arm fared better with respect to the chemotherapy-free interval (18.5 vs. 9.7 months; HR, 0.34; p < 0.0001) and time to the first subsequent therapy (16.7 vs. 7.7 months; HR, 0.35; p < 0.0001). OS was immature at the time of the analysis.
The PFS benefits observed in NORA were consistent with those reported in the NOVA trial, while the safety profile was indeed improved (Table 1). This particularly applied to hematological toxicities. Overall, the authors noted that niraparib at individualized starting doses is effective and safe and should be considered the standard clinical practice for maintenance therapy of patients with OC.
MEDIOLA: olaparib plus durvalumab ± bevacizumab
The combination of a VEGF inhibitor and olaparib has been shown to increase PFS compared with olaparib alone in patients with platinum-sensitive relapsed OC and compared with VEGF inhibition alone in the newly diagnosed maintenance setting [4, 5]. Initial results of the open-label, phase II basket trial MEDIOLA investigating olaparib plus durvalumab showed that the combination is well tolerated and active in patients with germline BRCA-mutant platinum-sensitive relapsed OC [6]. Two additional cohorts were sequentially enrolled to test olaparib plus durvalumab (n = 32) and olaparib plus durvalumab and bevacizumab (n = 31) in patients with germline BRCA-wildtype, platinum-sensitive relapsed OC after a maximum of 2 chemotherapy lines. Disease control rate (DCR) at 24 weeks and safety/tolerability were defined as the primary endpoints. Drew et al. presented the findings at the ESMO Congress [7].
According to this analysis, the chemotherapy-free triplet combination of olaparib, durvalumab and bevacizumab showed promising efficacy. At 24 weeks, DCR was high at 77.4 %, and median PFS amounted to 14.7 months. In the doublet combination cohort, 24-week DCR and PFS were 28.1 % and 5.5 %, respectively. Objective response rates were 87,1 % and 34.4 % for the triplet and doublet cohorts, respectively. An exploratory analysis suggested that the ORR achieved with the triplet regimen did not depend on the genomic instability status (GIS). GIS was positive by definition in patients with a loss of heterozygosity score ≥ 14, a somatic BRCA mutation or a mutation in one of 13 homologous recombination repair genes. The analysis showed consistently high response rates across the triplet cohort irrespective of GIS, indicating that the high ORR was not driven by differences in genomic instability status. Overall, the safety profiles of the doublet and triplet regimens matched the known safety profiles expected for the single agents. The combination of olaparib, durvalumab and bevacizumab is now being tested as part of a first-line maintenance regimen in the phase III DUO-O study.
Pivotal phase II results for novel agent pamiparib
The investigational, potent, selective, oral PARP1/2 inhibitor pamiparib has demonstrated antitumor activity in patients
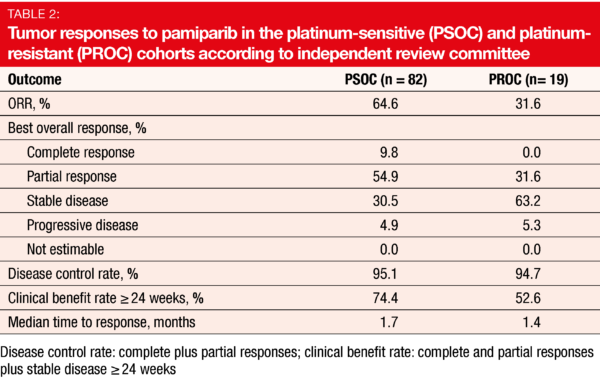
with OC in the first-in-human BGB-290-AU-002 study that also established 60 mg orally twice daily as the recommended phase II dose (RP2D) [8]. BGB-290-102, an open-label, multicenter phase I/II study, is assessing the safety and antitumor activity of pamiparib in adult Chinese patients with advanced solid tumors whose disease has progressed despite standard therapy or for which no standard therapy is available. Wu et al. reported preliminary results of the RP2D-expansion of the trial for OC patients with BRCA1/2-mutation-positive, platinum-sensitive (PSOC; n = 90) or platinum-resistant (PROC; n = 23) disease [9]. ORR according to independent review committee was defined as the primary endpoint.
Pamiparib gave rise to clinically meaningful and durable responses. Most of the patients in the PSOC cohort responded (ORR, 64.6 %), with 9.8 % achieving complete remissions (Table 2). Median duration of response was 14.5 months, and median PFS was 15.2 months. In the PROC group, ORR was 31.6 %. Disease control was obtained by 95.1 % and 94.7 % of patients in the PSOC and PROC cohorts, respectively. In both groups, most patients experienced reductions in their target lesions from baseline. The CA-125 response rates were 79.7 % and 38.1 %, respectively.
Pamiparib 60 mg twice daily was generally well tolerated and showed an acceptable safety profile. Similar to other PARP inhibitors, hematologic toxicities were the most significant safety events observed, although they proved manageable. The management of these AEs was optimized using a proactive modification plan and close monitoring. No myelodysplastic syndrome was reported, and no significant complications potentially related to hematologic toxicity (e.g., grade ≥ 3 hemorrhage, fever, infection) occurred. The overall safety profile was generally consistent across the PSOC and PROC cohorts.
REFERENCES
- Mirza MR et al., Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 2016; 375(22): 2154-2164
- Berek JS et al., Safety and dose modification for patients receiving niraparib. Ann Oncol 2018; 29(8): 1784-1792
- Wu X et al., Individualized starting dose of niraparib in patients with platinum-sensitive recurrent ovarian cancer (NORA): a randomized, double-blind, placebo-controlled, phase III trial. ESMO 2020, LBA29
- Liu et al., Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol 2014; 15(11): 1207-1214
- Ray-Coquard I et al., Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 2019; 381: 2416-2428
- Drew Y et al., An open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): Results in germline BRCA-mutated platinum-sensitive relapsed ovarian cancer. Gynecol Oncol 2018; 149 Suppl 1: 246-247
- Drew Y et al., Phase II study of olaparib plus durvalumab and bevacizumab (MEDIOLA): initial results in patients with non-germline BRCA-mutated platinum sensitive relapsed ovarian cancer. ESMO 2020, 814MO
- Lickliter J et al., Dose escalation/expansion study to investigate the safety, pharmacokinetics, food effect, and antitumor activity of BGB-290 in patients with advanced solid tumors. Ann Oncol 2017; 28(suppl 5): v123
- Wu X et al., Phase 2 study of pamiparib in Chinese patients with advanced ovarian Cancer. ESMO 2020, 820P
© 2020 Springer-Verlag GmbH, Impressum