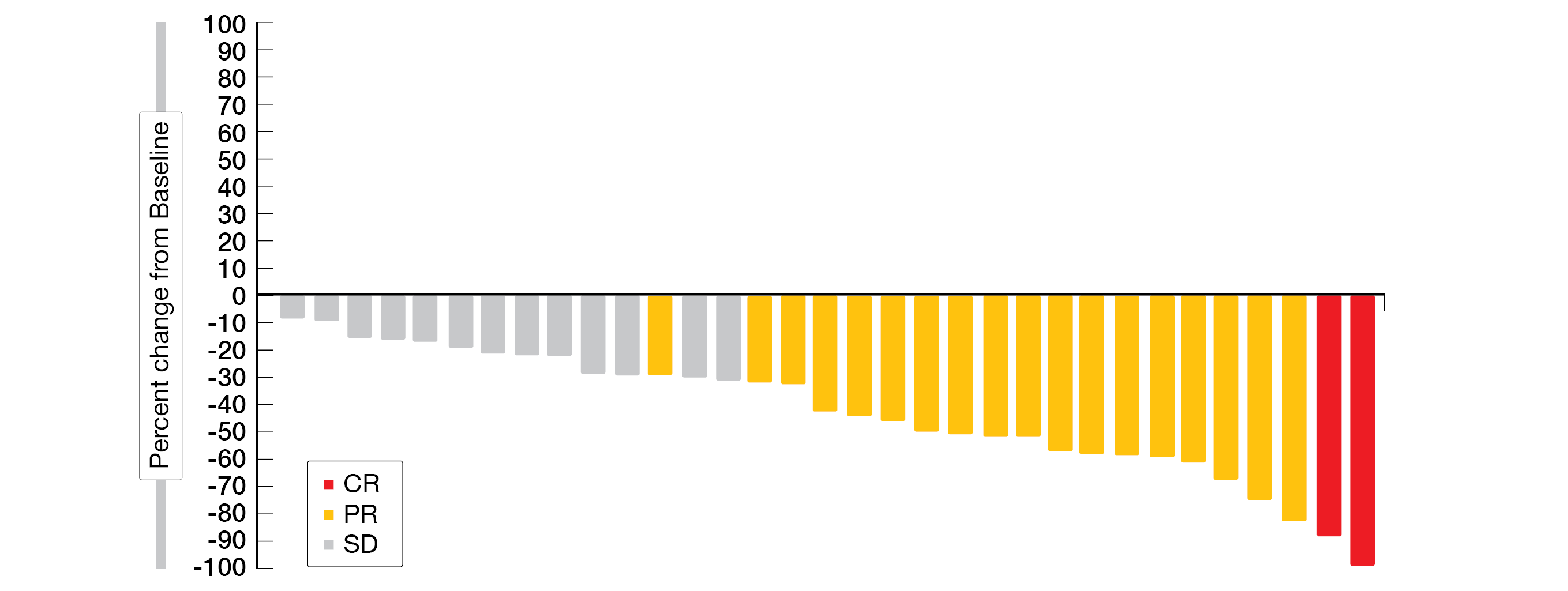From neoadjuvant to second-line therapeutic options for RCC patients
Kidney cancer accounted for more than 400,000 newly diagnosed cases in 2020, [1]. Interestingly, after over two decades of increasing rates, the worldwide incidence of renal cell carcinoma (RCC) has shown signs of plateauing in recent years, whereas an increase of the global kidney cancer death rate was observed. Here, the widespread use of non-invasive radiological techniques allows the detection of early and small RCCs, which are potentially curable [2].
Radical nephrectomy (RN) or partial nephrectomy (PN) are the standard-of-care for localized stage I to III non-metastatic RCC [2, 3]. Patients with stage II or III tumors have a substantial risk of post-nephrectomy relapse [4]. Therapeutic options in this patient population include sunitinib – a vascular endothelial growth factor (VEGF) pathway inhibitor approved in the US, only, based on the S-TRAC trial (NCT00375674) [5] – and pembrolizumab – an anti-PD-1 immune checkpoint inhibitor (ICI) approved in the US and the EU based on the KEYNOTE-564 trial (NCT03142334) [6]. Several clinical studies are currently ongoing to identify new effective adjuvant therapies and therefore improve efficacy outcomes.
CheckMate 914: dual ICI adjuvant therapy
The combination of the anti-PD-1 antibody nivolumab (NIVO) and the anti-CTLA4 antibody ipilimumab (IPI) has already demonstrated its significant superiority over sunitinib in terms of overall survival (median OS, 47.0 vs 26.6 months; HR, 0.68; 95 % CI, 0.58-0.81; p<0.0001) and duration of response (median DoR, not-reached [NR] vs 19.7 months; HR, 0.46; 95 % CI, 0.31-0.66; p<0.0001) in the CheckMate 214 trial in patients with untreated advanced or metastatic RCC [7, 8]. In a two-part trial, the combination NIVO+IPI is being assessed as adjuvant therapy in comparison to placebo (part A) or versus nivolumab monotherapy versus placebo (part B) in patients with resected stage II/III clear cell RCC in the phase III CheckMate 914 trial (NCT03138512). At this year’s ESMO conference, Motzer et al. reported on the efficacy and safety outcomes from part A of the CheckMate 914 trial [9].
Patients’ eligibility criteria included an RN or PN with negative surgical margins, a predominant clear cell histology, including sarcomatoid features as well as predefined tumor/node/metastasis TNM staging (pT2a grade 3/4 – N0, M0; pT2b, PT3 or pT4 any grade – N0, M0; or pT any grade – N1 M0), no residual or distant metastases after nephrectomy as confirmed by blinded independent central review (BICR) and a good performance status (ECOG 0-1). Four to 12 weeks after surgery, eligible patients were randomized 1:1 to receive either twelve cycles of nivolumab (240 mg, intravenously [IV], every second week [Q2W]) plus four cycles of ipilimumab (1 mg/kg, IV, Q6W) or equivalent placebo (twelve cycles of placebo [IV, Q2W] plus four cycles of placebo [IV, Q6W]). Patients were stratified by pathologic TNM stage and type of nephrectomy. The primary endpoint was disease free survival (DFS) by BIRC, while OS and safety were set as secondary endpoints.
A total of 816 patients were followed-up over a median period of 37 months. Patients’ median age was 58 years in the NIVO+IPI arm (n = 405) versus 57 in the placebo arm (n = 411). Most patients (93 % in each group) had a RN, and the most predominant tumor stage was pT3, G any, N0 M0 (78 % versus 77 %, respectively). The median duration of treatment was 5.1 months in both arms. The primary endpoint of DFS was not reached in the NIVO+IPI arm versus 50.7 months with placebo (HR, 0.92; 95 % CI, 0.71-1.19; p=0.5347). The 24-month DFS rate were 76.4 % for NIVO+IPI and 74.0 % for placebo, respectively. Due to a hierarchical testing procedure, and the fact that the DFS endpoint was no reached, no formal analysis of OS was performed. At the time of this analysis, 33 deaths were reported in the treatment arm and 28 deaths in the placebo arm.
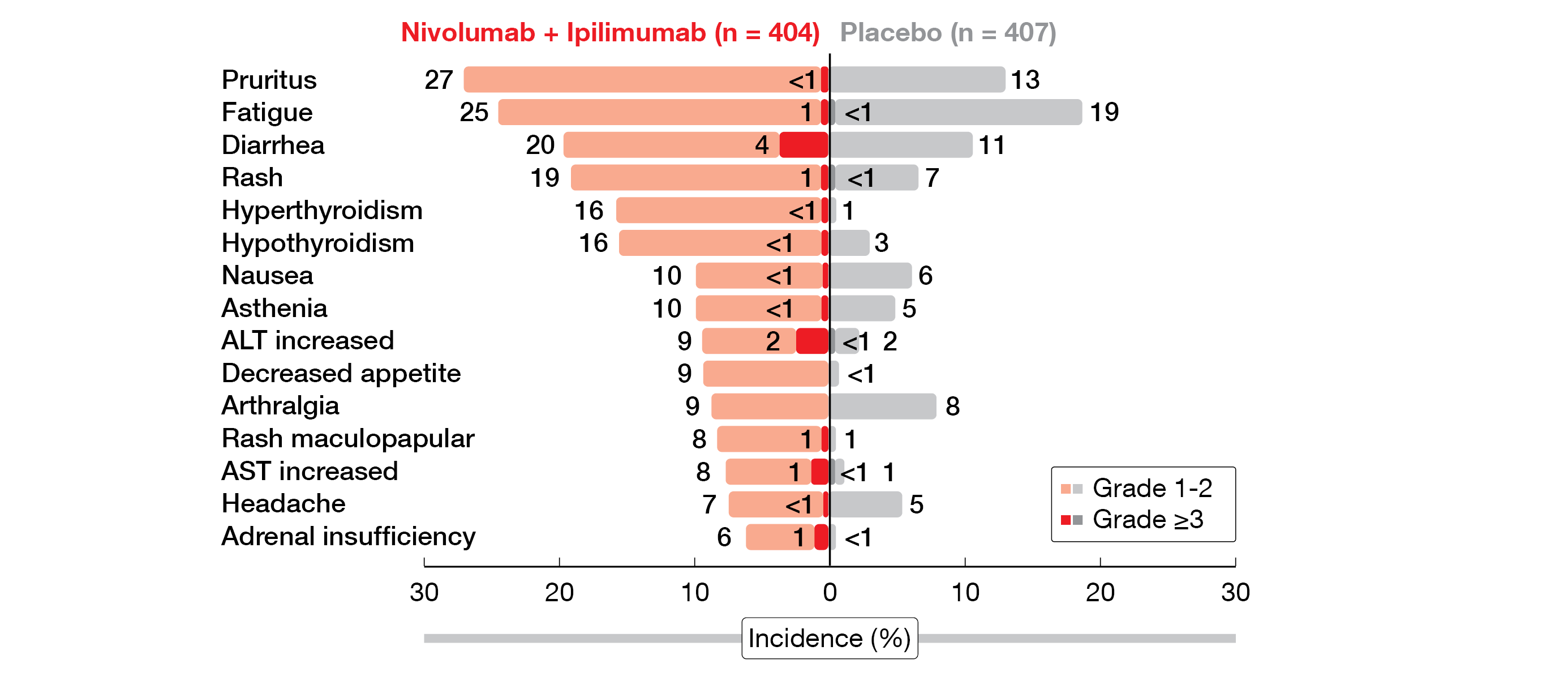
Overall, grade ≥3 treatment-related adverse events (TRAEs) in the NIVO+IPI arm were mild and included mostly diarrhea (4 %) and increased alanine transaminase (ALT, 2 %) (Figure 1). Four deaths (1 %) were reported due to drug toxicity. In total, 23 % of the patients in the combination arm versus 2 % in the placebo arm received corticosteroids to manage any grade immune-related AEs (irAEs), with diarrhea/colitis being the most frequent grade ≥3 irAE (5 %). Compared to placebo, more patients in the NIVO+IPI arm discontinued treatment (1 % vs 29 %) due to TRAEs any grade.
The combination of nivolumab plus ipilimumab did not meet the primary endpoint of DFS in patients with localized stage II/III RCC at high risk of post-nephrectomy relapse. Part B of CheckMate 914 focusing on nivolumab adjuvant monotherapy is currently ongoing.
Figure 1: Treatment-related AEs in all treated patients in the phase III CheckMate 914 trial.
IMmotion010 trial: adjuvant atezolizumab
The standard-of-care for patients with locoregional or oligometastatic RCC is PN or RN, with or without metastasectomy [10-12]. Atezolizumab, an anti-PD-L1 inhibitor that has already been approved in several malignant entities as adjuvant therapy, was investigated in patients with oligometastatic RCC after resection and increased risk of recurrence in the IMmotion010 phase III trial (NCT03024996). At ESMO 2022, Bex et al. presented the efficacy and safety outcomes [13].
Eligibility criteria were a resected intermediate to high-risk RCC, a predefined TNM tumor stage (pT2 grade 4, pT3a grade 3/4, pT3b/c or pT4 any grade, pTXN+ any grade, or M1 with no evidence of disease [NED]) and a clear cell or sarcomatoïd component. Patients were randomized 1:1 to receive either 16 cycles (or one year, whichever occurred first) of atezolimumab (1,200 mg, IV, Q3W) or of placebo (IV, Q3W). Patients were stratified by disease stage, PD-L1 expression of immune cell (IC) and geographic area. The primary endpoint was the investigator-assessed DFS in the intention-to-treat (ITT) population. Key secondary endpoints enclosed OS in the ITT population, investigator-assessed DFS in the IC1/2/3 population, independent review facility (IRF)-assessed DFS in the ITT and IC1/2/3 populations, IRF-assessed event-free survival (EFS) in the ITT population and safety.
From January 2017 to February 2019, 390 patients were enrolled in the treatment arm and 388 in the placebo arm; all of them were followed-up for more than 38.6 months. In both groups, the median age was approximately 60 years and almost 80 % of the patients had a very good performance status (ECOG 0). More than 90 % of the patients had a clear cell histology. The predominant tumor stage was pT2/PT3a (about 64 %) and the PD-L1 IC1/2/3 attained approx. 60 % in both arms. The primary endpoint was not met, as no DFS advantage was reported with atezolizumab compared to placebo (57.2 vs 49.5 months; HR, 0.93; 95 % CI, 0.75-1.15; p=0.495). This was seen in the DFS analysis of pre-specified subgroups, too, although there was a trend in favor of atezolizumab in females (HR, 0.61; 95 % CI, 0.40-0.94). Additionally, no improvement was observed with atezolizumab versus placebo in terms of median OS (NE in both groups; stratified HR, 0.97; 95 % CI, 0.67-1.42) or investigator-assessed DFS by PD-L1 status (PD-L1 IC expression < 1 % stratified HR, 1.09; 95 % CI, 0.77-1.59; PD-L1 IC expression 1 % to < 5 % stratified HR, 0.92; 95 % CI, 0.68-1.25; PD-L1 IC expression ≥ 5 % stratified HR, 0.57; 95 % CI, 0.29-1.15).
Atezolizumab was well tolerated, with a rate of grade 3-4 TRAEs of 14.1 % in the treatment arm versus 4.7 % in the placebo arm. The most frequent all-grade AEs were arthralgia (20 % with atezolizumab vs 14.9 % with placebo), pruritus (19.0 % vs 12.5 %) and hypothyroidism (14.4 % vs 3.1 %). AEs leading to discontinuation occurred in 11.5 % of the atezolizumab-treated patients versus 2.6 % in the control group.
In the IMmotion010 trial, atezolizumab did not improve clinical outcomes versus placebo as adjuvant therapy in patients with locoregional or oligometastatic RCC after resection and high risk of recurrence but adverse events were manageable and in line with the known safety profile of atezolizumab.
PROSPER, ECOG-ACRIN EA8143: neoadjuvant nivolumab
Several clinical investigations are currently evaluating neoadjuvant therapies to improve the survival of RCC patients undergoing nephrectomy by priming their immune system preoperatively. Preclinical studies demonstrated promising advantages of neoadjuvant immunotherapy over adjuvant therapy in eradicating metastatic disease [14]. This was confirmed in the clinical setting, with neoadjuvant nivolumab being associated with a good pathological response in lung, melanoma and breast cancer [15]. At this year’s ESMO conference Allaf et al. presented the interim analysis of a first phase III neoadjuvant study in RCC, the PROSPER, ECOG-ACRIN EA8143 trial (NCT03055013) [16].
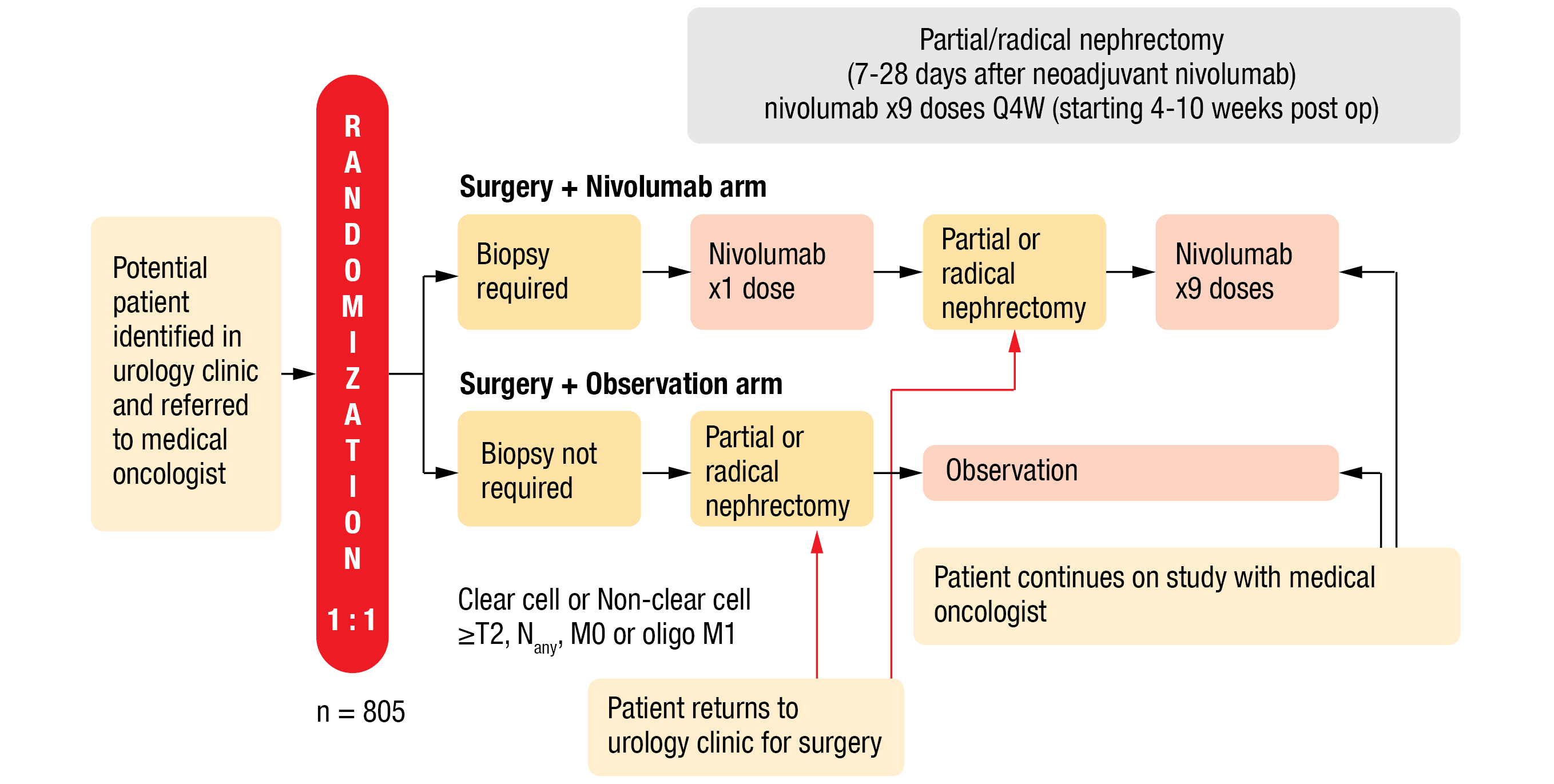
This open-label study compared neoadjuvant nivolumab followed by adjuvant nivolumab to observation in RCC patients undergoing nephrectomy. Eligible patients were randomized 1:1 to nivolumab or the observational arm. Inclusion criteria included a clear cell or non-clear cell histology and a minimum TNM tumor stage of ≥ T2, any N, M0 or oligo M1 (if resectable at the same time or within 12 weeks and patient rendered NED). Eligible patients in the treatment arm underwent biopsy, received one dose of nivolumab (480 mg, IV, Q4W) seven to 28 days before PN or RN, as well as nine adjuvant doses starting 4-10 weeks after surgery (Figure 2). The primary endpoint was recurrence-free survival (RFS), defined as time from randomization to disease recurrence or death, whichever occurred first. The secondary endpoints included OS, RFS for clear cell RCC (ccRCC), safety and tolerability, patient-reported outcomes, and correlative science.
A total of 819 patients were enrolled in the study and analyzed after a 16-month median follow-up. At baseline, about 50 % of patients presented with cT1/T2 tumors and the other half with cT3/T4 tumors. After surgery, more than 60 % of the patients had pT3/T4 tumors, 80 % had a ccRCC and about 3 % were not disease-free. At the time of data cut-off 87.4 % of patients had started neoadjuvant nivolumab, 88.9 % patients had received surgery and 77.7 % had started an adjuvant therapy in the treatment arm compared to 93.2 % of patients who had received surgery in the observational arm. This interim analysis did not demonstrate similar RFS in both arms (HR, 0.97; 95 % CI, 0.74-1.28, one-sided p-value=0.43) and the median RFS was not reached. The OS data were still immature when this interim analysis was performed but also did not differ significantly between the study groups (HR, 1.48; 95 % CI, 0.89-2.48; one-sided p=0.93). Therefore, ECOG-ACRIN data safety monitoring committee stopped the trial due to inefficacy.
More patients in the treatment arm than in the observational arm experienced grade 3-4 TRAEs (15 % vs 4 %) with the most common TRAEs being fatigue (60.4 % with nivolumab vs 26.6 % in the control arm), pruritus (31.5 % vs 2.8 %) and rash maculopapular (31.2 % vs 1.3 %). Treatment discontinuation due to any grade TRAEs occurred in 13 % of patients who received nivolumab.
This first phase III trial investigating the antitumoral activity and safety of perioperative nivolumab failed to meet its primary endpoint in RCC patients. However, nivolumab safety data were consistent with previous historic data. Future neoadjuvant RCC trials could be planned based on the ongoing radiomic, pathomic and biomarkers analyses of this pioneer trial.
Figure 2: Design of the PROSPER, ECOG-ACRIN EA8143 phase III trial.
COSMIC-313: 1L cabozantinib plus dual ICI
The combination of nivolumab plus ipilimumab is a first-line standard-of-care for advanced RCC of intermediate or poor risk, as defined by the International Metastatic RCC Database Consortium (IMDC) [17]. Although this combination has demonstrated OS superiority over sunitinib, a large proportion of patients (20 %) had a progressive disease as best response. Cabozantinib – a tyrosine kinase inhibitor (TKI) directed against a broad range of targets, including the VEGF receptor – already demonstrated its efficacy and safety as monotherapy [18-20] or in combination with nivolumab in advanced RCC [21]. The combination of cabozantinib plus nivolumab plus ipilimumab has been tested in phase I trials; it proved to be efficient and to have a manageable toxicity in patients with genitourinary tumors [22] or untreated advanced RCC [23]. At this year’s ESMO, Choueiri et al. reported on the COSMIC-313 phase III trial (NCT03937219), which currently evaluates this triple combination versus placebo in previously untreated patients with advanced RCC [24].
Study patients were treatment-naïve, had a clear cell histology, an IMDC intermediate or poor risk, a measurable disease as assessed by RECIST v1.1 and a minimum Karnofsky performance status ≥ 70 %. Eligible patients were randomized 1:1 to receive either cabozantinib (40 mg, per os[PO], every day [QD]) in arm A or placebo (PO, QD) in arm B plus nivolumab (3 mg/kg, IV, Q3W x 4) plus ipilimumab (1 mg/kg, IV, Q3W x 4), followed by cabozantinib (40 mg, PO, QD) plus nivolumab (3 mg/kg, IV, Q4W). The treatment was administered until loss of clinical benefit or intolerable toxicity. To note, no crossover was allowed. A tumor assessment according to RECIST v1.1 was planned every eight weeks. Patients were stratified by IMDC risk and region. The primary endpoint was PFS, as assessed by BICR per RECIST v1.1 in the PFS-ITT population (PITT). The secondary endpoint included OS in the ITT population, as well as the overall response rate (ORR), the DoR and safety.
Patients in the arm A (Cabo+Nivo+Ipi, n = 428) and in the arm B (Pbo+Nivo+Ipi, n = 427) were followed-up for a median duration of 17.7 months (ITT population, n = 855) or 20.2 months (PITT population, n = 550). Study participants had a median age of 60 to 61 years, a predominant intermediate IMDC risk (75 %) and two or more metastatic sites (80 %) mainly located in the lung (about 70 %) or in the lymph nodes (more than 50 %). The primary endpoint was met: the median PFS demonstrated a significant advantage of the cabozantinib combination compared to the control group in the PITT population (NR vs 11.3 months; HR, 0.73; 95 % CI, 0.57-0.94; p=0.013). In the subgroup analysis, the PFS by IMDC risk suggested a greater benefit for cabozantinib-treated patients having an intermediate risk (HR, 0.63; 95 % CI, 0.47–0.85) than those having a poor risk (HR, 1.04; 95 % CI, 0.65-1.69). At the data cut-off (January 31, 2022), the ORR in the PITT population was also improved in the Cabo+Nivo+Ipi arm with 43 % (3 % with a complete response [CR], 41 % with a partial response [PR]) compared to the Pbo+Nivo+Ipi arm with 36 % (3 % CR, 32 % PR), while the disease control rate (DCR) reached 86 % and 72 %, respectively. Compared to the control arm, a higher ORR benefit was observed in patients with an intermediate risk (45 % vs 35 %) receiving the cabozantinib-combined therapy than in those who presented with a poor risk (37 % vs 38 %).
Overall, grade 3-4 TRAEs were more frequent in the arm A than in the arm B (73 % vs 41 %), with the most common ones being increased ALT (26 % vs 6 %, respectively), increased aspartate aminotransferase (AST, 20 % vs 5 %), increased lipase (9 % vs 6 %) and hypertension (8 % vs 2 %). Three patients experienced grade 5 TRAEs within 30 days after the last dose in each arm.
The phase III COSMIC-313 trial was the first study to use a combination of two standard-of-care ICIs as control arm (nivolumab plus ipilimumab). This trial demonstrated efficacy and safety of cabozantinib plus dual ICI over placebo plus dual ICI in previously untreated patients with advanced RCC of IMDC intermediate or poor risk. Further followed-up to determine overall survival is still ongoing.
LITESPARK-003: belzutifan plus cabozantinib
Belzutifan is a highly selective small molecule designed to inhibit the hypoxia-inducing factor (HIF)-2α that is a key oncogenic driver in ccRCC, when it is constitutively activated. Belzutifan has previously shown antitumoral activity with an ORR of 25 % in heavily pretreated patients with ccRCC (NCT02974738) [25]. As cabozantinib was already approved for advanced ccRCC as monotherapy [18-20], a combined treatment targeting both the HIF-2α and VEGF pathway might improve patients’ outcome in advanced ccRCC even further.
At ESMO 2022, Merchan et al. presented the interim analysis of the cohort 1 of the LITESPARK-003 phase II trial (NCT03634540) [26]. Key eligibility criteria include locally advanced or metastatic ccRCC with no prior treatment and a good performance status (ECOG 0 or 1). Tumor assessments are planned at week 9, then Q8W until month 12, and Q12W thereafter. The primary endpoint is the ORR, as assessed per RECIST v1.1by the investigator; PFS, DoR and time to response (TTR) assessed per RECIST v1.1 by investigator, OS, as well as safety and tolerability, are secondarily analyzed.
At the time of data cut-off (February 1, 2022), 35 out of 50 planned treatment-naïve patients had been enrolled with a median followed-up of 14 months. Median age was 64 years, 83 % males, 60 % of patients had a good performance status (ECOG 0) and 40 % presented with an intermediate or poor IMDC risk. Eligible patients receive belzutifan (120 mg, PO, QD) plus cabozantinib (60 mg, PO, QD).
More than half of the patients responded to the treatment (ORR, 57 % [6 % CR, 51 % PR]); DCR, 94 %). To note, the ORR was consistent across the IMDC favorable versus intermediate/poor risk categories (62 % [10 % CR, 52 % PR] vs 50 % [50 % PR]). Most patients (94 %) experienced a reduction in target lesion size (Figure 3). At the time of this interim analysis, 25 patients (71 %) were still on treatment. The median TTR was 1.9 months (range, 1.7-9.2) and the median DoR was 28.6 months (range, 1.7+ to 28.6), with 78 % of responders having a DoR of at least twelve months. The median PFS was 30.3 months (95 % CI, 9.4-NR), the 1-year PFS rate was 67 % and the 1-year OS rate was 96 %.
Grade 3 TRAES were experienced by 37 % of the patients, while no grade 4 or 5 TRAEs were reported. Serious TRAEs occurred in 6 % of patients. Anemia and diarrhea (71 %, each) and fatigue (63 %) were the most frequent TRAEs any grade. One patient discontinued cabozantinib due to an AE (abdominal abscess) whereas no patient discontinued belzutifan due to an AE. Twenty-five (71 %) patients experienced belzutifan-related anemia (including two grade 3 events) and two patients (6 %) experienced belzutifan-related hypoxia (including one grade 3 event).
Moreover, at ESMO 2022, McDermott et al. presented an update from cohort 2 of LITESPARK-003 including patients with prior immunotherapy and a maximum of two regimens for locally advanced or metastatic RCC [27]. At the time of data cut-off (February 1, 2022), 10 of 52 patients were still on treatment. Median age was 63 years, 73 % males, 56 % of patients had a ECOG 1. Most patients had one prior line of anticancer therapy (56 %) with more patients being treated with immunotherapy only (54 %) compared to anti-VEGF/VEGFR therapy (46 %).
With a median follow-up of 24.6 months, belzutifan plus cabozantinib still showed a promising antitumor activity in patients with advanced ccRCC previously treated with immunotherapy (ORR, 31 % [2 % CR, 29 % PR]). To note, the ORR was consistent across the different pre-treatment groups – immunotherapy only (ORR, 32 %), immunotherapy/anti-VEGF therapy (ORR 29 %), 1 line of prior therapy (ORR, 31%) and 2 lines of prior therapy (ORR, 30 %). Most patients (87 %) experienced a reduction in target size with a median DoR of 18.6 months (range, 4.2+ to 22.8). The median PFS was 13.8 months (range, 9.2-19.4) and the 1-year PFS rate was 56 %. The median OS was 24.1 months (range, 20.0-37.4) and the 1-year OS rate was 77 %. The safety profile was consistent with individual profiles of each agent and no grad 4 TRAEs occurred. Of note, one patient died due to treatment-related respiratory failure.
The interim outcomes of the LITESPARK-003 phase II trial showed manageable safety accompanied by promising antitumor activity of belzutifan plus cabozantinib in treatment-naïve as well as previously immunotherapy-treated patients with advanced ccRCC. Thus, data from the ongoing phase III study LITESPARK-011 trial (NCT04586231) are highly awaited.
Figure 3: Best percentage change from baseline in target lesions of treatment naïve-patients in the LITESPARK-003 phase II trial. CR, complete response; PR, partial response; SD, stable disease.
REFERENCES
- Sung, H, et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71(3): 209-249.
- Escudier, B, et al., Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol 2019; 30(5): 706-720.
- Motzer, RJ, et al., Kidney Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022; 20(1): 71-90.
- Motzer, RJ, et al., Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients With Localized or Locally Advanced Renal Cell Carcinoma. J Clin Oncol 2017; 35(35): 3916-3923.
- Ravaud, A, et al., Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N Engl J Med 2016; 375(23): 2246-2254.
- Choueiri, TK, et al., Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. Reply. N Engl J Med 2021; 385(20): 1920.
- Motzer, RJ, et al., Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer 2022; 128(11): 2085-2097.
- Motzer, RJ, et al., Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 2019; 20(10): 1370-1385.
- Motzer, RJ, et al., Adjuvant nivolumab plus ipilimumab (NIVO+IPI) vs placebo (PBO) for localized renal cell carcinoma (RCC) at high risk of relapse after nephrectomy: Results from the randomized, phase III CheckMate 914 trial. Ann Oncol 2022; 33(suppl 7; Abstr LBA4).
- Powles, T, et al., ESMO Clinical Practice Guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Ann Oncol 2021; 32(12): 1511-1519.
- Ljungberg, B, et al., European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur Urol 2022; 82(4): 399-410.
- Ingels, A, et al., Complementary roles of surgery and systemic treatment in clear cell renal cell carcinoma. Nat Rev Urol 2022; 19(7): 391-418.
- Bex, A, et al., IMmotion010: Efficacy and safety from the phase III study of atezolizumab (atezo) vs placebo (pbo) as adjuvant therapy in patients with renal cell carcinoma (RCC) at increased risk of recurrence after resection Ann Oncol 2022(suppl 7; Abstr LBA66).
- Liu, J, et al., Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov 2016; 6(12): 1382-1399.
- Forde, PM, et al., Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018; 379(9): e14.
- Allaf, M, et al., Phase III randomized study comparing perioperative nivolumab (nivo) versus observation in patients (Pts) with renal cell carcinoma (RCC) undergoing nephrectomy (PROSPER, ECOG-ACRIN EA8143), a National Clinical Trials Network trial. Ann Oncol 2022; 33(suppl 7; Abstr LBA67).
- Motzer, RJ, et al., Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 2018; 378(14): 1277-1290.
- Choueiri, TK, et al., Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015; 373( 19): 1814-1823.
- Choueiri, TK, et al., Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016; 17(7): 917-927.
- Choueiri, TK, et al., Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. Eur J Cancer 2018; 94: 115-125.
- Choueiri, TK, et al., Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2021; 384(9): 829-841.
- Apolo, AB, et al., Phase I Study of Cabozantinib and Nivolumab Alone or With Ipilimumab for Advanced or Metastatic Urothelial Carcinoma and Other Genitourinary Tumors. J Clin Oncol 2020; 38(31): 3672-3684.
- Escudier, B, Nivolumab + Ipilimumab + Cabozantinib for Previously Untreated Advanced RCC: Results from a Discontinued Study Arm of CheckMate 9ER. EIKCS 2022 2021; Abs 1.
- Choueiri, TK, et al., Phase III study of cabozantinib (C) in combination with nivolumab (N) and ipilimumab (I) in previously untreated advanced renal cell carcinoma (aRCC) of IMDC intermediate or poor risk (COSMIC-313). Ann Oncol 2022(supp 7; Abstr LBA8).
- Bauer, T, et al., The oral HIF-2 α inhibitor MK-6482 in patients with advanced clear cell renal cell carcinoma (RCC): updated follow-up of a phase I/II study. Genitourinary Cancers Symposium 2021; February 11-13, 2021: Abstract 273.
- Choueiri, TK, et al., Phase II study of belzutifan plus cabozantinib as first-line treatment of advanced renal cell carcinoma (RCC): Cohort 1 of LITESPARK-003. Ann Oncol 2022; 33(supp 7; Abstr 1447O).
- McDermott, DF, et al., Phase II study of belzutifan plus cabozantinib for previously treated advanced renal cell carcinoma (RCC): Update from cohort 2 of LITESPARK-003. Ann Oncol 2022; 33(supp 7; Abstr 1453P)
© 2022 Springer-Verlag GmbH, Impressum