Immunotherapy-based treatment of Hodgkin lymphoma: What is new?
Second-line pembrolizumab in addition to GVD
Immune-checkpoint blockade plays a key role in the therapeutic landscape of relapsed or refractory classical Hodgkin lymphoma (r/r cHL). Nevertheless, novel strategies to enhance responses remain of clinical interest. In the second-line setting, the phase II study presented by Moskowitz et al. at ISHL 2022 evaluated pembrolizumab in addition to up to four cycles of gemcitabine, vinorelbine, and liposomal doxorubicin (GVD) [1]. Thirty-nine patients were enrolled. The primary endpoint was complete response (CR) after 2–4 cycles. Patients who achieved CR after two cycles were eligible for autologous stem cell transplantation (ASCT).
In the group of 38 evaluable patients, 92 % (n = 35) obtained CR after two cycles of pembrolizumab plus GVD. Including one additional patient achieving CR after two more cycles, the CR rate for this regimen was 95 %. ASCT was performed in 36 patients, and consolidation with brentuximab vedotin post ASCT was conducted in 14 cases. Only one patient in the entire study relapsed. At a median follow-up of 32.2 months, all of the 38 evaluable patients responded, and 95 % were in CR. The 30-month progression-free survival (PFS) rate was 96 %. A second cohort was added that received four cycles of pembrolizumab plus GVD followed by 13 doses of pembrolizumab maintenance without transplant in case of CR. To date, CR has been achieved by 88 % of the 34 individuals enrolled. Across the two cohorts (n = 73), 96 % of patients responded to treatment, and 92 % obtained CR.
The most common adverse events (AEs) included rash, transaminase elevations, oral mucositis, nausea and fatigue, with the majority being grade 1 or 2. One patient died from pneumonitis, and another recovered from grade 4 sepsis. The authors recommended a low threshold to recognize and treat engraftment syndrome. Taken together, these results contribute to the data pool that shows high CR rates and durable remissions with PD-1 blockade plus chemotherapy followed by ASCT. Further research is required regarding the optimal salvage regimen, the selection of patients with r/r cHL who need transplant, and the question of whether transplant can be moved safely to the third line or beyond.
Brentuximab vedotin plus nivo, ipi, or both
The phase I E4412 study reported by Diefenbach et al. assessed the antibody-drug conjugate brentuximab vedotin (BV) in combination with either ipilimumab (n = 21), nivolumab (n = 18), or both (n = 22) in patients with relapsed HL [2]. The three treatment groups were enrolled sequentially. Each group contained three arms. Arms A-C were treated with BV plus ipilimumab Q6W, arms D-F received BV plus nivolumab Q3W, and arms G-I were treated with BV plus nivolumab Q3W and ipilimumab Q12W. The study was based on the hypothesis that while the immune checkpoint inhibitors might abrogate tumor tolerance in T cells, BV would directly target the cancer cells.
Common AEs included peripheral sensory neuropathy, nausea, diarrhea, and fatigue; these were primarily grade 1 and 2. Among serious AEs, maculo-papular rash prevailed. One case of grade 4 Stevens-Johnson syndrome occurred, as well as two cases of grade 5 pneumonitis in the nivolumab-treated arms. No late immunologic toxicities were observed.
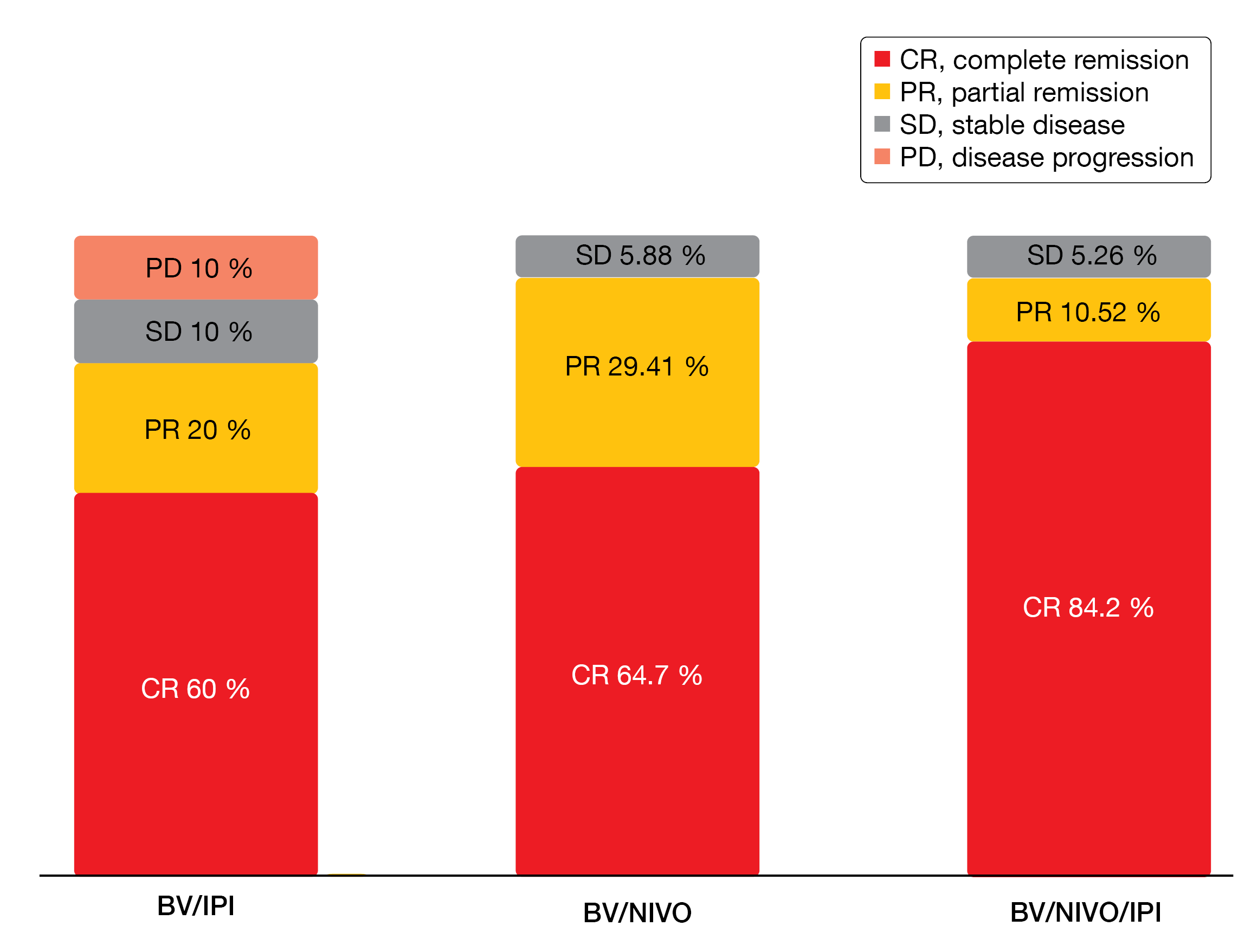
In the response-evaluable group, CRs resulted in 60 % with BV/ipilimumab, 64.7 % with BV/nivolumab, and 84.2 % with the triple combination (Figure 1). After a median follow-up of 2.5 years, median duration of response was 1 year for BV/ipilimumab and had not been reached with either BV/nivolumab or BV/nivolumab/ipilimumab. Thus, both nivolumab-containing regimens appeared to be superior to the ipilimumab-based regimen. Similarly, median PFS was shorter with BV/ipilimumab (1.10 years) than with BV/nivolumab (not reached) and BV/nivolumab/ipilimumab (2.49 years). Nevertheless, even the patients in the BV/ipilimumab arm who relapsed were alive at 3 years. Patients achieving CR had longer median PFS (not reached) than those with partial response (PR; 1.8 years). Activity of the nivolumab-based regimens was also seen in eight BV-pretreated patients, with no qualitative differences in terms of depth of response or PFS compared to the BV-naïve group.
Future questions relate to the use of doublet vs. triplet therapies and potential superiority of post-transplant PFS compared to PFS with standard second-line treatment. Also, patients might be identified who have low-risk disease and can defer ASCT. The phase II E4412 trial has concluded adult enrollment, and pediatric enrollment is ongoing.
Figure 1: Response rates for brentuximab vedotin plus ipilimumab, nivolumab, or both in the response-evaluable population
Anti-LAG-3 treatment in the anti-PD-1–refractory setting
Although PD-1 inhibitors are a standard of care in patients with r/r cHL, optimal treatment after failure of immunotherapy needs to be fully defined. LAG-3 is involved in T-cell regulation and is commonly co-expressed with PD-1 on anergic T cells [3]. Dual blockade of PD-1 and LAG-3 has already been shown to exert antitumor activity in patients with unresectable or metastatic melanoma [4].
The multicohort, open-label, phase I/II MK-4280-003 trial was designed to assess the anti-LAG-3 antibody favezelimab in combination with pembrolizumab in patients with relapsed/refractory hematologic malignancies including cHL, DLBCL, and indolent NHL. Part 1 included all cohorts and was the safety lead-in phase to determine the recommended phase II dose, which was favezelimab 800 mg plus pembrolizumab 200 mg Q3W. Only one dose-limiting toxicity (i.e., autoimmune hepatitis grade 3) was found at the favezelimab 200-mg dose level. No dose-limiting toxicities emerged with the favezelimab 800-mg dose. In the dose-expansion phase (part 2), the patients received the combination at the established dose for ≤ 35 cycles.
At ISHL 2022, Timmerman et al. presented results for cohort 2 that contained 34 anti-PD-1–refractory patients with r/r cHL [5]. These had experienced disease progression after PD-1–targeted therapy and had either relapsed after ASCT or were ineligible for it, or had displayed no response to salvage chemotherapy. Favezelimab plus pembrolizumab showed a manageable safety profile. After a median follow-up of 18.2 months, hypothyroidism was the most frequently observed AE (18 %), followed by fatigue and nausea (15 % each) as well as arthralgia and diarrhea (12 % each). All of these AEs were grade 1 or 2. Grade 3 or 4 treatment-related AEs (TRAEs) occurred in 18 %. No patient died due to treatment-related events. Treatment was discontinued in 18 %.
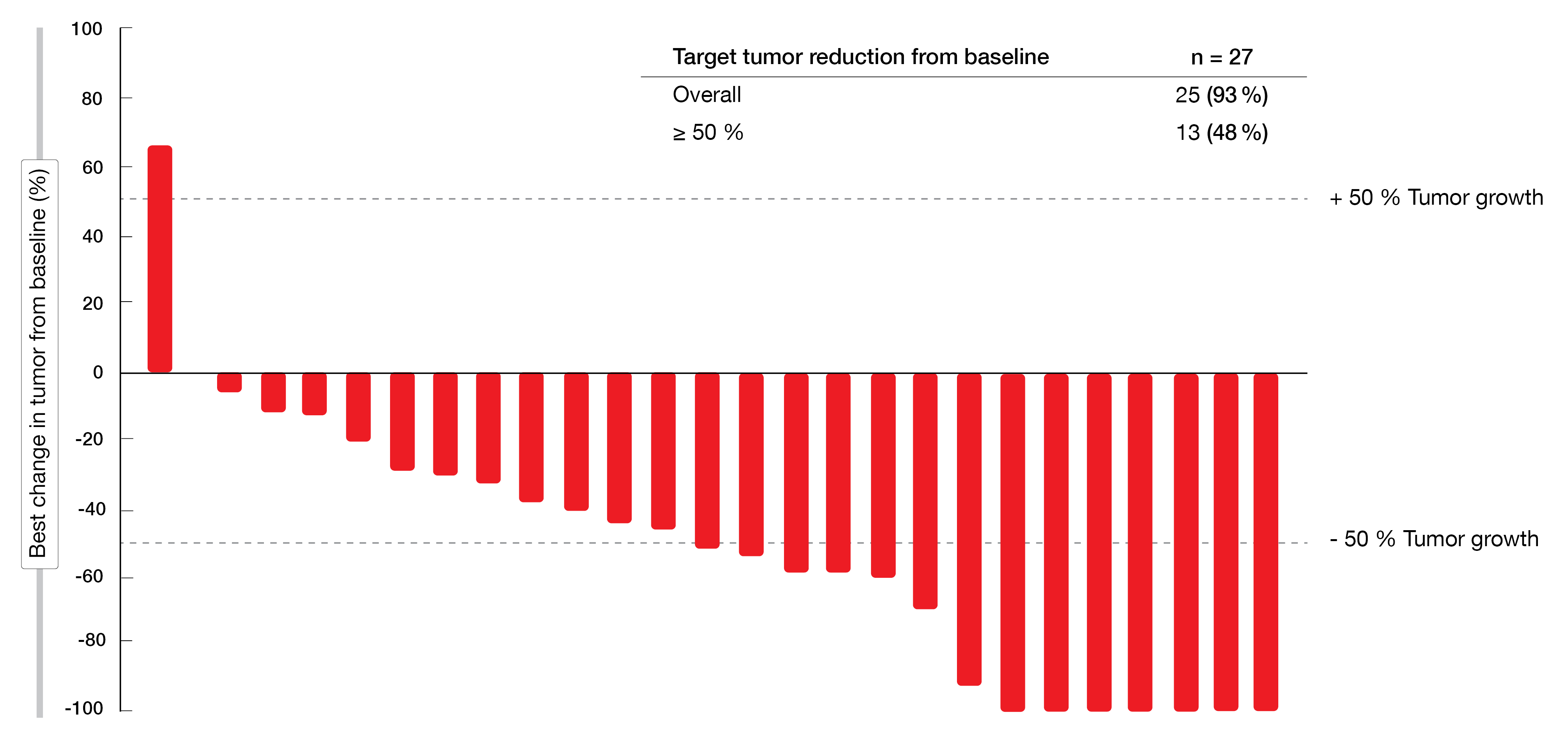
The analysis demonstrated promising activity of favezelimab plus pembrolizumab. Overall, 93 % of 27 patients who had both baseline and post-dose assessments at the time of data cutoff derived reductions in their target lesions, with 48 % experiencing shrinkage of ≥ 50 % (Figure 2). Ten patients responded, which resulted in an overall response rate (ORR) of 30 %, and 3 (9 %) achieved CR. The responses lasted for a median of 19.4 months. Median PFS and overall survival (OS) were 9.4 and 25.7 months, respectively. A phase III trial investigating a coformulation of favezelimab/pembrolizumab in patients with cHL refractory to anti–PD-1 therapy is ongoing (NCT05508867).
Figure 2: Target tumor reduction obtained with pembrolizumab plus favezelimab
Favezelimab & pembrolizumab in the anti-PD-1–naïve group
Borchmann et al. reported findings for cohort 1 of the MK-4280-003 study that consisted of anti-PD-1–naïve patients with r/r cHL (n = 30) [6]. In this group, 87 % had TRAEs, the most common being hypothyroidism (27 %), infusion-related reactions (23 %), and fatigue (20 %). Grade 3 or 4 TRAEs occurred in 23 %. Thirteen percent of patients discontinued treatment due to TRAEs; no fatal treatment-related events were noted.
After a median follow-up of 14.1 months, the ORR was 73 %, with a CR rate of 27 %. Almost all patients who received post-dose scans experienced reductions in their target lesions. Median OS and median duration of response had not been reached yet; 55 % of patients responded for ≥ 12 months. Median PFS was 19.4 months. The authors emphasized in their conclusion that favezelimab 800 mg plus pembrolizumab 200 mg Q3W demonstrated acceptable safety and antitumor activity in anti-PD-1–naïve patients with r/r cHL. Studies comparing favezelimab with single-agent pembrolizumab might be of interest.
Fixed-dose vs. weight-adjusted nivolumab
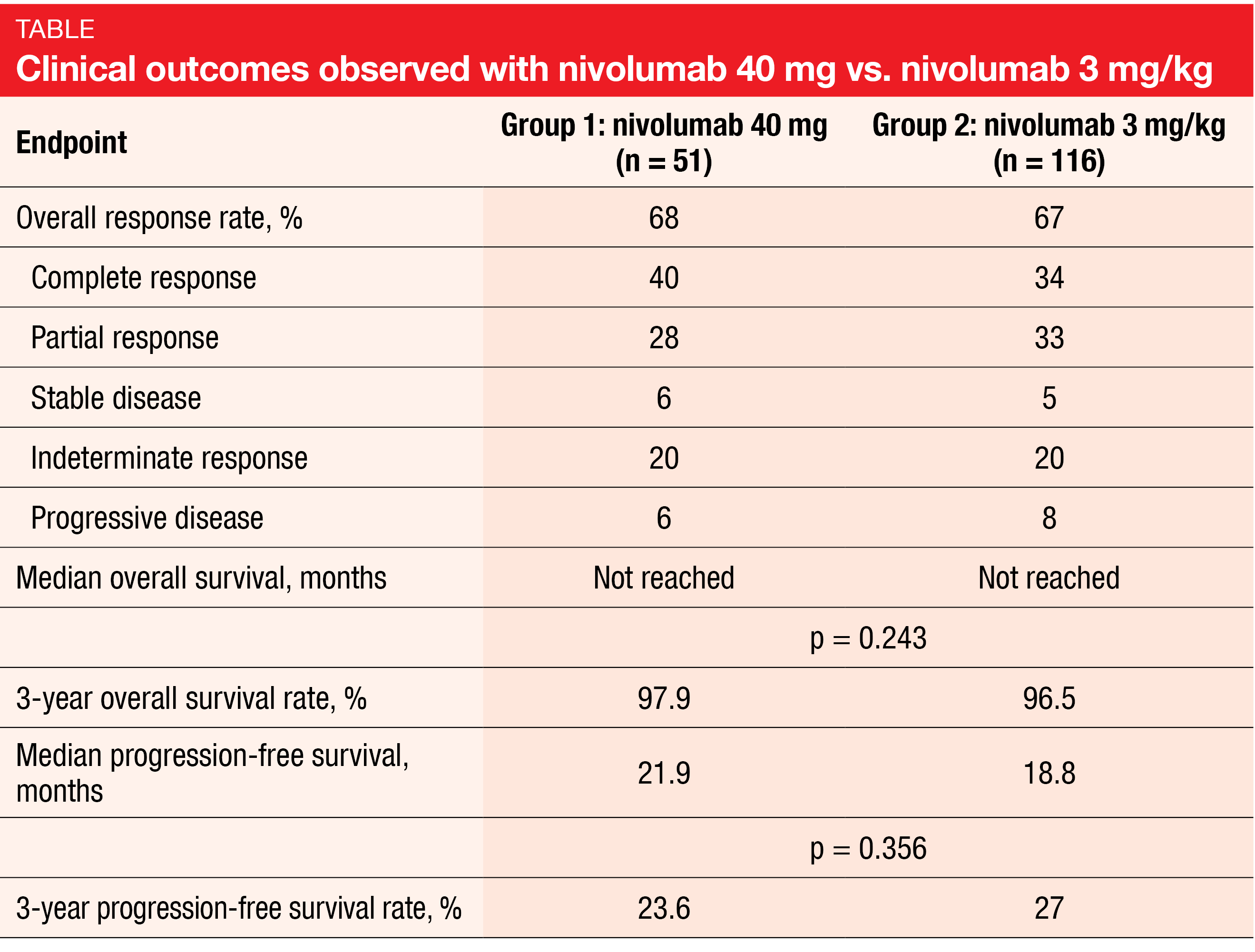
Although the introduction of nivolumab has changed the landscape of r/r cHL treatment, this therapy remains inaccessible for a significant number of patients worldwide, especially in low-income countries, due to its high cost. Low-dose nivolumab administered at a fixed dose of 40 mg was explored in the Nivo40 trial relative to the standard dosing regimen of 3 mg/kg in patients with r/r cHL [7]. An expanded prospective fixed-dose cohort of patients (group 1, n = 51) was compared to the retrospective group 2 (n = 116) that received nivolumab 3 mg/kg.
After a median follow-up of 48 and 60 months in groups 1 and 2, respectively, the ORRs were similar (68 % vs. 67 %; Table), as was the timing of best response to treatment (cycle 6 in both groups). Likewise, median PFS did not differ across the groups (21.9 vs. 18.8 months; p = 0.356), and median OS had not been reached yet with either treatment. Any-grade AEs were observed in 65 % vs. 79 % (p = 0.068), with grade 3/4 AEs occurring 10 % vs. 19 % (p = 0.151). Additional therapies after nivolumab monotherapy were initiated in 78 % vs. 84 %. Ten percent vs. 22 % of patients underwent allogeneic SCT. Overall, the data demonstrated comparable efficacy of fixed-dose nivolumab treatment versus the standard dose of 3 mg/kg, although final statements require a direct comparison in the prospective setting.
Prognosis after nivolumab discontinuation
Fedorova et al. retrospectively analyzed the survival of patients with r/r cHL after discontinuation of nivolumab, as well as the efficacy and safety of retreatment [8]. Among the 48 patients included, 29 and 19 had been treated with nivolumab 3 mg/kg and 40 mg, respectively. They had discontinued nivolumab due to different reasons in CR (n = 40) or PR (n = 8).
After a median follow of 48 months, disease relapse was observed in 63 %. In the group that had achieved CR, median PFS was 24 months, with a 4-year PFS rate of 43.6 %. Patients who had obtained PR showed a median PFS of 7.7 months; all of these progressed. One person died due to progressive multifocal leukoencephalopathy after allogeneic stem cell transplantation.
Retreatment with single-agent nivolumab was initiated in 22 patients. At a median follow-up of 33 months, the ORR was 73 %, with CR and PR resulting in 41 % and 32 %, respectively, and indeterminate response in 27 %. Median PFS was 24.8 months; at 3 years, 26.7 % of patients were progression-free. AEs occurred in 41 % during retreatment, and grade 3/4 AEs were noted in 18 %. According to the authors’ conclusion, patients with r/r cHL who have achieved CR with nivolumab therapy might experience durable remission after treatment discontinuation. In case of disease relapse, nivolumab retreatment appears to represent an effective and safe option.
ctDNA monitoring of first-line APVD
In the setting of untreated cHL, the combined administration of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) is a standard treatment option. A single-arm pilot study explored the combination of pembrolizumab with doxorubicin, vinblastine, and dacarbazine (APVD) as front-line treatment in 30 patients with any-stage cHL. Interim PET/CT assessment was performed after two cycles of APVD, followed by another PET/CT scan at the end of treatment, i.e., after a maximum of four additional cycles. The total duration of treatment depended on determinants such as disease stage and risk factors. According to findings presented in 2021, CR was achieved in 66 % after two cycles of pembrolizumab 200 mg Q3W and AVD in standard dosing Q2W [9]. The analysis demonstrated durable remission, with a 2-year PFS rate of 97 %. Only one progression event had occurred within two years.
The prognostic role of interim PET/CT is limited in patients treated with ABVD, while the role of interim and end-of-treatment PET with immune checkpoint combinations is undefined. Lynch et al. hypothesized that circulating tumor DNA (ctDNA) monitoring with phased variant enrichment and detection sequencing (PhasED-seq) might improve response assessment in patients receiving APVD [10]. PhasED-seq, as compared to other ctDNA profiling methods, has the ability to reduce error rates, particularly at low cell-free DNA levels [11].
Higher undetectable ctDNA rates vs. PET
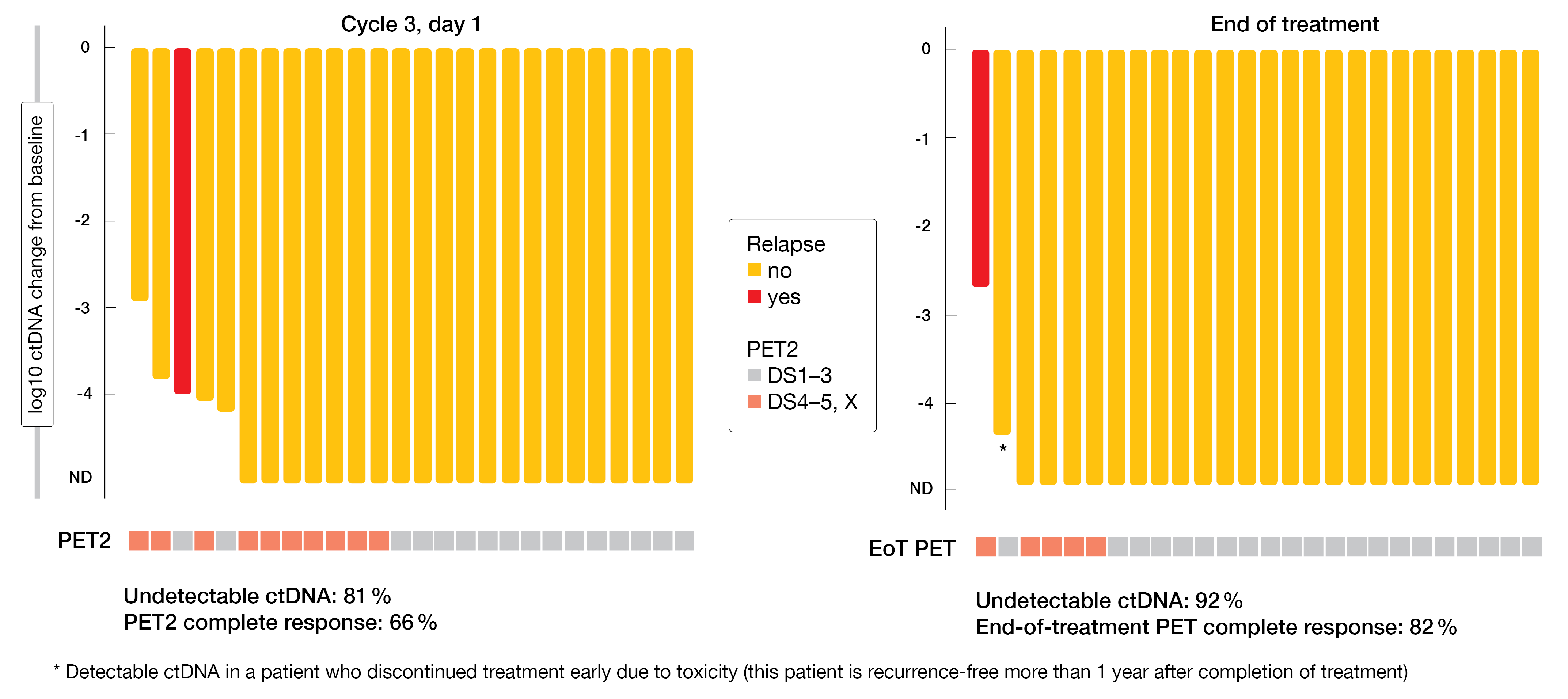
Minimal residual disease tracking was possible in almost all patients from the single-arm pilot study (n = 26). The data showed that pretreatment ctDNA levels reflected the tumor burden; correlations were seen with disease stage at diagnosis and the total metabolic tumor volume. On day 1 of cycle 3, ctDNA was undetectable in 81 % (Figure 3). At that time, the PET CR rate was 66 %. Undetectable ctDNA increased to 92 % at the end of treatment, with the PET CR rate rising to 82 %. One patient relapsed, and four had positive end-of-treatment PET despite undetectable ctDNA, although none of them recurred up to 36 months after completion of treatment. In light of these findings, the utility of FDG-PET with APVD remains undefined for the time being, as the authors noted.
According to a post-hoc analysis, ctDNA clearance was associated with improved PFS both at cycle 3 (p = 0.025) and end of treatment (p = 0.0016). Moreover, the results implied that ctDNA might be a more sensitive and specific measure of residual disease than PET. Residual ctDNA was detected in a progressing patient showing complete metabolic response according to PET, whereas another patient with residual FDG uptake 3 months after the end of treatment has remained ctDNA-negative and progression-free for several years. The authors suggested that PhasED-Seq integrated with conventional imaging might improve accuracy of response assessment. Twenty additional patients with advanced-stage disease have been added to enrich for individuals with higher risk.
Figure 3: Log reduction in ctDNA and PET complete response rates at cycle 3 as well as end of treatment with pembrolizumab plus AVD
Pembrolizumab plus chemotherapy in pediatric patients
Slow early response (SER) to initial chemotherapy and added radiotherapy exposure increases the risk of relapse and toxicity in patients with cHL. The open-label, phase II KEYNOTE-667 study evaluated pembrolizumab plus chemotherapy in pediatric patients and young adults with cHL and SER. Interim data were presented at the ISHL 2022 for group 2 (n = 30) that had newly diagnosed, high-risk, stage IIEB to IVB cHL (age range, 3–25) [12]. After two cycles of vincristine, etoposide, prednisone/prednisolone and doxorubicin, those with SER (i.e., Deauville score 4 or 5) were treated with ≤ 17 doses of pembrolizumab 2 mg/kg to 200 mg (3–17 years) or 200 mg (18–25 years) Q3W plus four cycles of cyclophosphamide, vincristine, prednisone/prednisolone, and dacarbazine (COPDAC-28). Patients who remained PET-positive and had SER after COPDAC-28 consolidation underwent radiotherapy plus ≤ 17 doses of pembrolizumab. On the other hand, those with SER who turned PET-negative received only pembrolizumab for ≤ 17 doses. The primary end point was ORR by blinded independent central review in patients with SER.
The data suggested that pembrolizumab might enhance responses to chemotherapy in this population. Among the patients who had a post-COPDAC-28 assessment, 68 % were PET-negative and thus did not require irradiation. Pembrolizumab plus COPDAC-28 demonstrated acceptable safety. In 47 %, TRAEs were reported, and 7 % had TRAEs grade ≥ 3. One patient developed grade 2 immune-mediated hypothyroidism.
Trial in progress: individualized immunotherapy
In the setting of early-stage unfavorable HL, the randomized phase II GHSG NIVAHL trial has shown feasibility, a favorable safety profile and outstanding efficacy of nivolumab plus first-line chemotherapy followed by involved-site radiotherapy (IS-RT) [13]. According to the most recent update, the 2-year PFS and OS rates were 99 % and 100 %, respectively [14].
The upcoming open-label, GHSG phase II INDIE trial will investigate an individualized immunotherapy approach with the anti–PD-1 antibody tislelizumab in the same setting (NCT04837859) [15]. At 35 GHSG trial sites in Germany, patients with newly diagnosed early-stage unfavorable HL will receive two doses of tislelizumab 200mg Q3W followed by restaging and response-adapted further treatment, i.e., either four additional tislelizumab doses or four cycles of tislelizumab plus chemotherapy. The main cohort of 100 patients aged 18–60 years will undergo consolidative IS-RT in case of PET-positive residues only. Thus, both radiotherapy and chemotherapy can potentially be omitted in optimally responding individuals. The study endpoints including PFS, OS, feasibility, safety, patient-reported outcomes and correlative endpoints will allow for insights into response-adapted first-line immunotherapy.
REFERENCES
- Moskowitz A et al., Phase II study of pembrolizumab plus GVD as second-line therapy for relapsed or refractory classical Hodgkin lymphoma. ISHL 2022, T099
- Diefenbach CS et al., Extended follow-up of a phase I trial of ipilimumab, nivolumab and brentuximab vedotin in relapsed Hodgkin lymphoma: A trial of the ECOG-ACRIN Research Group (E4412). ISHL 2022, T100
- Woo SR et al., Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 2012; 72(4): 917-927
- Tawbi HA et al., Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med 2022; 386(1): 23-34
- Timmerman J et al., Safety and dose-expansion study of combination favezelimab (anti-LAG-3) plus pembrolizumab in patients with relapsed or refractory classical Hodgkin lymphoma refractory to anti-PD-1 treatment. ISHL 2022, T058
- Borchmann P et al., Safety and dose-expansion study of combination favezelimab (Anti-LAG-3) plus pembrolizumab in anti-PD-1-naïve patients with relapsed or refractory classical Hodgkin lymphoma. ISHL 2022, P061
- Fedorova L et al., The efficacy and safety of nivolumab 40 mg therapy versus 3 mg/kg in patients with relapsed and refractory classic Hodgkin lymphoma. ISHL 2022, P062
- Fedorova L et al., Prognosis of patients with relapsed and refractory classic Hodgkin lymphoma after nivolumab discontinuation and efficacy of nivolumab retreatment. ISHL 2022, P060
- Lynch RC et al., Concurrent pembrolizumab with AVD for untreated classical Hodgkin lymphoma. Blood 2021; 138(suppl 1): 233
- Lynch RC et al., Circulating tumor DNA in classical Hodgkin lymphoma patients treated with pembrolizumab and chemotherapy: dynamic response assessment and correlation with baseline total metabolic tumor volume. ISHL 2022, T057
- Kurtz DM et al., Enhanced detection of minimal residual disease by targeted sequencing of phased variants in circulating tumor DNA. Nat Biotechnol 2021; 39(12): 1537-1547
- Vinti L et al., Pembrolizumab in pediatric patients and young adults with newly diagnosed classical Hodgkin lymphoma and slow early responders to frontline chemotherapy: the phase 2 KEYNOTE-667 study. ISHL 2022, P089
- Bröckelmann PJ et al., Efficacy of nivolumab and AVD in early-stage unfavorable classic Hodgkin lymphoma: The randomized phase 2 German Hodgkin Study Group NIVAHL trial. JAMA Oncol 2020; 6(6): 872-880
- Bröckelmann PJ et al., Efficacy and safety of nivolumab and AVD in early-stage unfavorable Hodgkin lymphoma: Extended follow-up from the GHSG phase II Nivahl trial. ASH 2020, 1153
- Bröckelmann PJ et al., Trial in progress: Individualized immunotherapy in early-stage unfavorable Hodgkin lymphoma – the investigator-initiated phase II GHSG INDIE trial. ISHL 2022, P063
© 2022 Springer-Verlag GmbH, Impressum
More posts
Immunotherapy-based treatment of Hodgkin lymphoma: What is new?
Immunotherapy-based treatment of Hodgkin lymphoma: What is new? Second-line pembrolizu
Preface – ISHL 2022
Preface – ISHL 2022 ©MSKCC - Alison J. Moskowitz, MD, Memorial Sloan Kettering, Cance