Emergent BTKi treatments in WM
Additionally to ibrutinib, to date the only once-daily BTK inhibitor approved in the USA and the European Union either as monotherapy or in combination with RTX for patients with WM [1], other BTKis, such as acalabrutinib and zanubrutinib, are now emerging as potential therapeutic alternatives.
Acalabrutinib in treatment-naïve or R/R patients
Acalabrutinib is an emergent, potent, and selective BTKi, which has received accelerated approval by the US FDA for the treatment of adult patients with relapsed or refractory (R/R) MCL and is in clinical development for CLL and DLBCL. Roger Owen presented the updated data of the phase II clinical trial (NCT02180724) evaluating acalabrutinib activity and safety in the treatment of treatment naïve (TN) or R/R patients with WM after a five-year follow-up [2, 3].
Exclusion criteria included a prior BTKi therapy, a significant cardiovascular disease, comorbidities (such as organ system dysfunction or uncontrolled active systemic infection) or requiring treatment with vitamin K, antagonists, or proton-pump inhibitors. Enrolled patients were administered with acalabrutinib (100 mg twice a day [BID] or 200 mg per day [QD] per os [PO]) for 28-day cycles until disease progression (PD) or unacceptable toxicity. The primary endpoint was the ORR as assessed by investigator, while key secondary endpoints were duration of response (DoR), PFS, OS and safety.
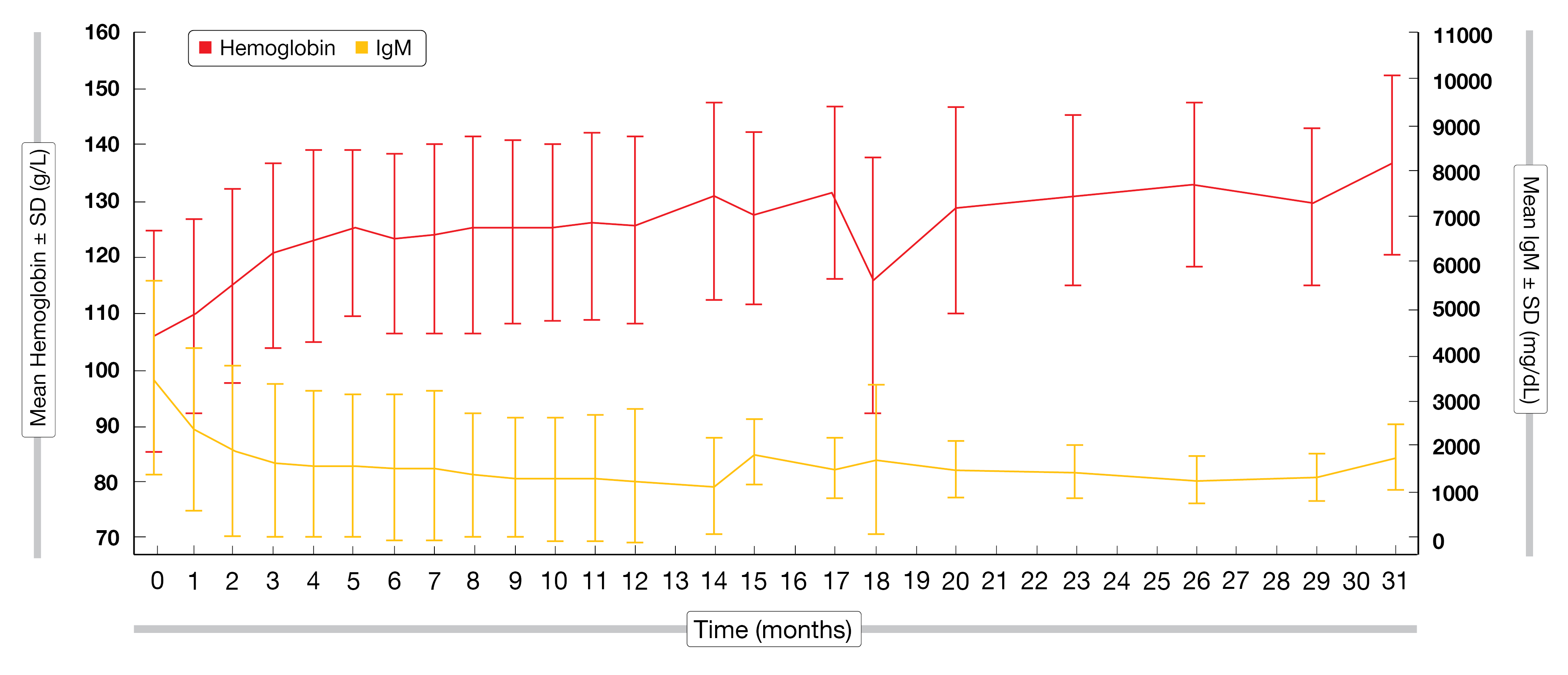
Overall, 106 patients were enrolled across 27 sites in the USA and across Europe from September 2014 to December 2015. The median follow-up time was 63.7 months at the time of data cut-off. Patients in the R/R disease cohort (n = 92) were slightly younger than in the TN cohort (n = 14) (median age, 69 vs 73 years). Patients in the R/R cohort had a median of two prior treatments, 45 % of them had at least three prior treatments, and most of them (87 %) had been exposed to anti-CD20 therapy. One third of the R/R cohort was refractory to the last therapy. According to the modified 3rd iwWM criteria, the ORR in the R/R cohort reached 95 %, with a MRR of 82 % (VGPR, 41 %; PR, 38 %; MR, 13 %). In the TN cohort, the ORR was 93 % and the MRR 79 %, including a VGPR of 7 %. The treatment resulted in clinical benefits for patients, with a prompt decrease in IgM levels and Hgb improving at a rapid rate (Figure 1). The median PFS for the whole cohort was 67.5 months, with an estimated 66-month PFS rate of 52 % in the R/R cohort and of 84 % in the TN cohort and an estimated 66-month OS rate of 71 % and 91 %, respectively. Multivariate analysis showed that having two prior lines of therapy had a significant negative impact on the PFS rate compared to two or less therapies (53.7 % vs 83.5 %; HR = 1.892; 95% CI, 1.007-3.557; p = 0.0441).
There were more AEs in the TN cohort than in the R/R cohort (29 % vs 16 %). Disease progression was reported in 7 % in the TN cohort and 22 % in the R/R cohort. The most common grade 3-4 AEs were infections (33% in the R/R cohort and 14 % in the TN cohort) and cardiac events (7 % and 14 %, respectively). Grade 3-4 bleeding (7 %), atrial fibrillation (2 %) and hypertension (4 %) were observed in the R/R group only.
This phase II trial demonstrated the high efficiency of acalabrutinib in treating WM, with a confirmed long-term efficacy in the R/R setting. The toxicity profile was favorable, with a relatively low discontinuation rate and a good cardiovascular profile. Further clinical evaluation of acalabrutinib in randomized controlled trials are warranted.
Figure 1: Mean hemoglobin and IgM levels of patients in the R/R disease cohort.
Long-term follow-up of zanubrutinib
Zanubrutinib, another potent next-generation BTKi, shows a similar efficacy and a higher selectivity compared to ibrutinib. Zanubrutinib has specifically been designed to maximize BTK occupancy. Its advantageous pharmacokinetic (PK) and pharmacodynamic properties allow a median of 24-hour occupancy of 100 % with BID dosing in peripheral blood mononuclear cell (PBMC) and lymph nodes [4]. Moreover, zanubrutinib showed a reduced off-target inhibition of other tyrosine kinases family members [5]. Besides, zanubrutinib can be administered along with strong/moderate cytochrome P3A (CYP3A) inhibitors at a reduced dose, as well as proton pump inhibitors, acid-reducing agents, and antithrombotic agents [6].
Zanubrutinib safety, PK, antitumor activity, and optimal dosing in B-cell malignancies including WM have been assessed in the phase 1/2 BGB-3111-AU-003 trial [7]. Safety and efficacy data of the 3.5-year follow-up analysis were presented at iwWM 2022 by Judith Trotman [8].
Initially, this international trial included patients with R/R disease, then broadened up to include TN patients in phase II of the study. Eligibility criteria included having a WHO-defined B cell malignancy, at least one prior therapy (in the R/R cohort), no available higher-priority treatment, an ECOG PS scoring of two or less, a minimal absolute neutrophil count of 1000/µL and a minimal platelet count of 100,000/µL. Other eligibility criteria enclosed having an adequate renal and hepatic function, as well as no significant cardiac disease. The dose escalation phase consisted of 40, 80 and 160 mg of zanubrutinib QD, followed by the dose expansion phase consisting of 320 mg QD or 160 mg BID.
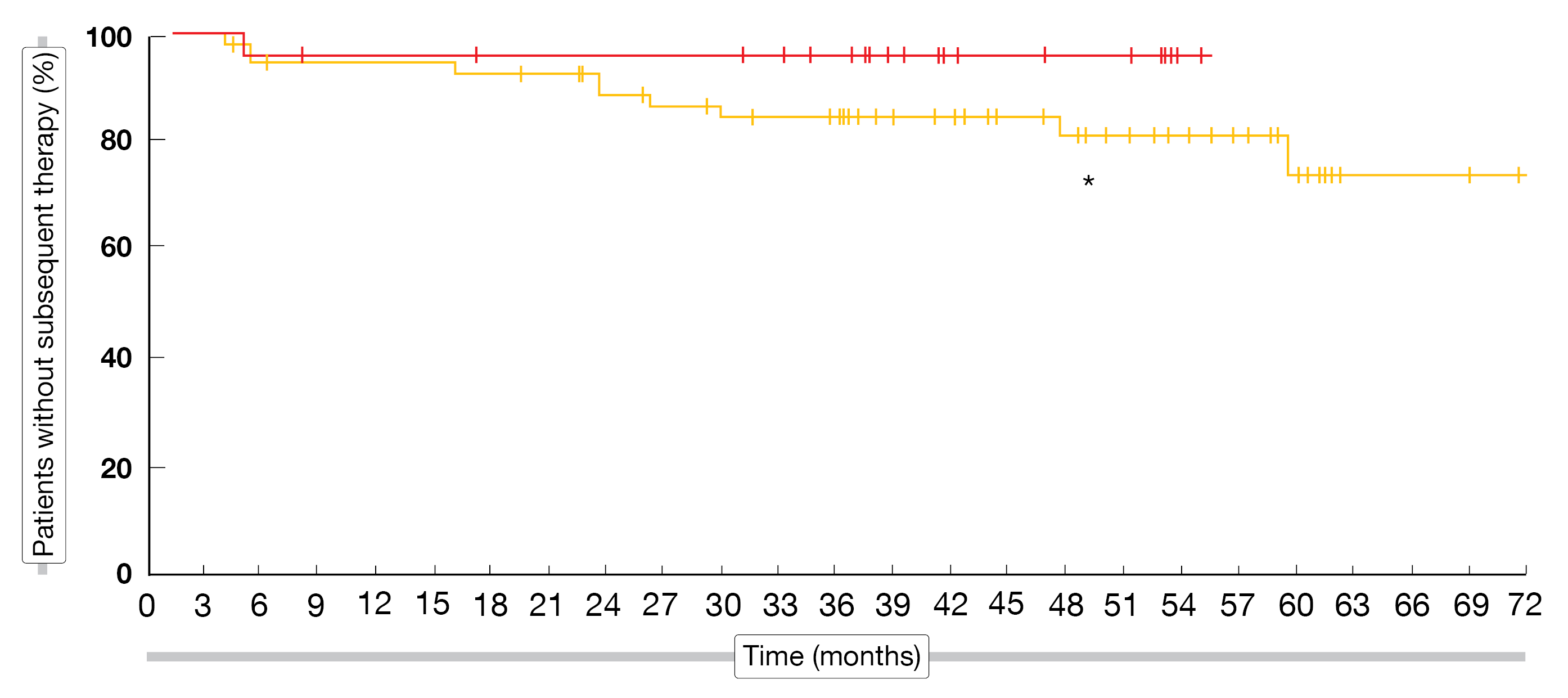
Amongst the 78 WM patients recruited, 73 were evaluable for efficacy, 32 discontinued due to PD or toxicity and 46 rolled-over to long-term extension study. Patients’ genotype slightly differed amongst R/R and TN patients (MYD88MUT, 72 % vs 83 %). Most patients responded to the treatment (ORR = 96 %) and the major response rate reached 82 %. VGPR and CR were attained by 49 % and 2 % of the R/R patients, as well as 33 % and 4 % of the TN population. To note, there was a comparable response rate between the QD (n = 22, ORR = 91 %, VGPR + CR rate = 32 %) and BID (n = 47, ORR = 98 %, VGPR + CR rates = 49 %) doses. The median follow-up of R/R patients was 48.8 months compared to 39.6 months in the TN population. The 4-year PFS rate was higher in the TN population (71.2 % vs 64.7 %). Of note, the rate of patients without subsequent therapy plateaued over a median of three years, just below 100 %, in the TN population and over 80 % of the R/R patients have not moved on to the next line of treatment either (Figure 2).
All WM patients experienced TEAEs; 64 % of them had grade ≥ 3 TEAEs. Most common grade ≥ 3 AEs were infections (29.5 %), neutropenia (16.7 %), second primary malignancies (12.8 %) and anemia (11.5 %). In total, 17 % of the AEs led to a treatment discontinuation, with 3.8 % of them being related to zanubrutinib. A comparable safety was observed regardless of the dosage timing (QD or BID).
Long-term treatment with zanubrutinib was very well tolerated and resulted in durable responses. Deep responses were observed in both TN and R/R patients, as well as in all molecular subtypes including MYD88WT. There was no apparent exposure safety and efficacy relationships between the QD and BID groups, which allowed for extrapolation despite the small number of patients treated QD. Both regimens have been approved for WM by US Food and Drug Administration (FDA), Health Canada, Australia, and European Medical Agency (EMA).
Figure 2: Time to next treatment in patients with TN or R/R WM. *, median follow-up
Long-term data of zanubrutinib therapy in a Chinese R/R population
Zanubrutinib efficacy and safety have been evaluated in Chinese patients with R/R WM in the phase II BGB-3111-210 trial (NCT03332173) [9]. Shuhua Yi presented the long-term analysis of this multicenter trial after a median follow-up of 33 months at this year’s iwWM meeting [10].
Patients included in this study were over 18-year old, had a confirmed WM pathology, met at least one treatment criteria according to the 7th iwWM panel consensus [11], had received at least one prior line of standard CIT regimen and documented failure to achieve MR of disease progression (PD). Patients received zanubrutinib monotherapy (160 mg, PO, BID) until PD or intolerable toxicity. The primary endpoint was MRR rate (CR + VGPR + PR) as assessed by an independent review committee according to the response criteria updated at the 6th iwWM [12]. PFS, ORR, duration of major response (DoMR) and safety were set as secondary endpoints.
A total of 44 patients were recruited, whose median age was 65 years and ECOG performance status was 0/1 for most of them (93.2 %). Three quarters had an intermediate or high-risk WM prognostic score, and they had received a median of two prior therapies. The rate of patients with MYD88WT was surprisingly high (15.9 %) in this cohort. Peripheral blood cytopenia at study enrollment were frequent (anemia 75 %, thrombocytopenia 20.5 % and neutropenia 25 %).
The MRR attained 69.8 % (VGPR, 32.6 %; PR, 37.2 %) and was close to the ORR of 76.7 % (MR or better). After a median follow-up of 33 months, the median PFS and DoMR rates were not reached. The 18- and 24-month PFS rates were 68.1 % and 60.5 %, respectively, while the 18- and 24-month DoMR rates both reached 75.1 %. The median time to major or overall response was 2.8 months each, and the median time to VGPR or CR was 4.2 months. The subgroup analysis confirmed the MRR benefit of zanubrutinib in all items analyzed – including MYD88WT and CXCR4MUT genotypes. However, the best MRR was obtained within the MYD88MUT/CXCR4WT WM patients.
The rate of grade ≥ 3 TEAEs or serious TEAEs was 77.3 % and 56.8 %, respectively. Overall, 11.4 % of TEAEs led to a treatment discontinuation and 4.5 % of them led to death. No atrial fibrillation or flutter events were reported.
After a 33-month follow-up, administrating zanubrutinib to Chinese patients with R/R WM proved to be an effective treatment, as demonstrated by the high rate of deep, rapid, and durable response. The safety profile and tolerability of zanubrutinib was satisfactory and comparable to prior study results.
REFERENCES
- Imbruvica, Summary of product characteristics 2022; Available from: https://www.ema.europa.eu/en/documents/product-information/imbruvica-epar-product-information_en.pdf.
- Owen, RG, et al., Acalabrutinib monotherapy in patients with Waldenström macroglobulinemia: a single-arm, multicentre, phase 2 study. Lancet Haematol 2020; 7(2): e112-e121.
- Owen, R, et al., Long Term Follow-up-Acalabrutinib Phase II. 2022; iwWM 2022, Session 10.
- Tam, CS, et al., Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 2019; 134(11): 851-859.
- Guo, Y, et al., Discovery of Zanubrutinib (BGB-3111), a Novel, Potent, and Selective Covalent Inhibitor of Bruton’s Tyrosine Kinase. J Med Chem 2019; 62(17): 7923-7940.
- Mu, S, et al., Effect of rifampin and itraconazole on the pharmacokinetics of zanubrutinib (a Bruton’s tyrosine kinase inhibitor) in Asian and non-Asian healthy subjects. Cancer Chemother Pharmacol 2020; 85(2): 391-399.
- Trotman, J, et al., Zanubrutinib for the treatment of patients with Waldenström macroglobulinemia: 3 years of follow-up. Blood 2020; 136(18): 2027-2037.
- Trotman, J, et al., Long Term Follow-Up Zanubrutinib-Phase II. 2022; iwWM 2022, Session 10.
- An, G, et al., A Phase II Trial of the Bruton Tyrosine-Kinase Inhibitor Zanubrutinib (BGB-3111) in Patients with Relapsed/Refractory Waldenström Macroglobulinemia. Clin Cancer Res 2021; 27(20): 5492-5501.
- Yi, S, et al., Extended follow-up of a phase 2 trial of te Bruton tyrosine kinase inhibitor zanubrutinib (BGB-3111) in Chinese patients with relapsed/refractory Waldenström Macroglobulinemia. 2022; iwWM 2022, Session 10.
- Dimopoulos, MA, et al., Treatment recommendations for patients with Waldenström macroglobulinemia (WM) and related disorders: IWWM-7 consensus. Blood 2014; 124(9): 1404-1411.
- Owen, RG, et al., Response assessment in Waldenström macroglobulinaemia: update from the VIth International Workshop. Br J Haematol 2013; 160(2): 171-176.
© 2023 Springer-Verlag GmbH, Impressum
More posts
Emergent BTKi treatments in WM
Emergent BTKi treatments in WM Additionally to ibrutinib, to date the only once-daily B
BTK inhibition in Waldenström’s macroglobulinemia: trial updates and biomarker analysis
BTK inhibition in Waldenström’s macroglobulinemia: trial updates and biomarker analysis
Management of WM patients previously exposed to BTK-inhibitors
Management of WM patients previously exposed to BTK-inhibitors Zanubrutinib in ibrutin
New insights into BTKi treatment of Waldenström‘s macroglobulinemia
New insights into BTKi treatment of Waldenström‘s macroglobulinemia New insights into
Preface – iwWM 2022
Preface – iwWM 2022 © author’s own - Efstathios Kastritis, MD, Plasma Cell Dyscrasia