Latest updates on BTKi-treatment in WM
Long-term BTKi monotherapy
Ibrutinib monotherapy is approved for all lines of therapy in patients with WM, although its initial trial was focused on patients who relapsed, only (NCT01614821) [1]. Thus, clinical research on the use of ibrutinib monotherapy in the frontline setting of WM is warranted. At this year’s iwWM congress, Jorge Castillo presented long term data (4 years) of ibrutinib monotherapy in treatment-naïve WM patients [2].
In this investigator-initiated, open-label, prospective phase II study, single-agent ibrutinib (420 mg once daily) was evaluated in treatment-naive patients with WM (NCT02604511) [3]. At the data cut-off date (March 16, 2021), 31 patients were allocated to treatment intervention and 19 patients completed the study. The median patients’ age was 63 to 64 years and about half of them had a CXCR4 mutation (CXCR4MUT, n = 14; CXCR4WT, n = 16). Both groups presented with similar characteristics, although CXCR4MUT patients had significantly lower rates of adenopathies (14 % vs 50 %, p = 0.04) and lower β2-microglobulin levels (3.4 mg/L vs 4.2 mg/L, p = 0.07) than CXCR4WT patients.
Ibrutinib-treatment induced a 100 % response rate, including 87 % of patients with a major response. Between CXCR4MUT and CXCR4WT patients, there was a slight difference in major response (78 % vs 94 %; p = 0.09) and a numerically, though not significant, lower VGPR rate in patients with than without CXCR4 mutations (14% vs. 44%). Median time to ibrutinib response (TTR) was 0.9 months in CXCR4WT and a little longer in CXCR4MUT patients (1.7 months). Median time to major response (TTMR) was significantly shorter in CXCR4WT (1.8 vs 7.3 months, p = 0.01). The 4-year progression-free survival (PFS) rate was 76 % for the overall study population, 59 % in patients with a CXCR4 mutation and 92 % in patients without CXCR4 mutation (p = 0.06).
The most frequent grade ≥3 adverse events (AEs) included elevation of alanine aminotransferase (ALT), hypertension and neutropenia (in 3 patients each). Patients also experienced anemia, rash, urinary tract infection (in 2 patients each); one case of grade 4 cardiac arrest and one case of grade 4 thrombocytopenia were also reported. Of note, six patients experienced atrial fibrillation (grade 2). In all cases, therapy was further administered during the management of AEs.
Ibrutinib monotherapy, as frontline treatment in patients with WM was associated with rapid, deep, and durable responses without unexpected adverse events. While ibrutinib responses were affected by CXCR4 mutation, long-term disease control was attained regardless of CXCR4 mutational status.
INNOVATE study: BTKi combined therapy
The INNOVATE trial (NCT02165397) was designed to evaluate the efficacy of the combination ibrutinib plus rituximab (RTX) for the treatment of patients with WM [4]; this combination was not yet approved at the time of trial initiation. Data from a primary analysis with a median follow-up of 26.5 months previously demonstrated significant higher PFS rates with this combination compared to placebo plus RTX [4]. Additionally, outcomes of recent real-world studies confirmed the clinical trial results [5, 6]. At iwWM 2022 meeting, Christian Buske presented the final analysis of the INNOVATE trial after a 63-month median follow-up [7] .
INNOVATE is a large international, prospective, randomized phase III trial. Patients’ eligibility criteria included confirmed WM, a measurable disease (serum IgM > 0.5 g/dL), and a sensitivity to RTX. Patients were randomized 1:1 and allocated to either experimental arm A (ibrutinib per os [PO], 420 mg, once daily until progressive disease (PD) plus RTX intravenously [IV] 375 mg/m2 on Day 1 of Weeks 1-4 and 17-20) or to arm B (placebo until PD plus RTX, same administration and dosage as in arm A). Patients in arm B were allowed to crossover to single-agent ibrutinib after progression. Patients were stratified according to International Prognostic Scoring System for WM (IPSSWM), number of prior regimens and ECOG performance status (PS). The primary endpoint was PFS, and secondary endpoints enclosed response rates as assessed by an independent central review committee (ICR), OS, Hbg improvement, time to next treatment (TTNT) and safety. At study closure, patients without PD were allowed to further receive ibrutinib in an extension program.
Median age was 70 years in arm A (n= 75) and 68 years in arm B (n= 75), and IPSSWM was intermediate or high for around 80 % of the patients. Although most patients had received one or two prior systemic therapies, there was a substantial proportion of treatment-naïve patients (45 %) in each arm. About 45 % of the patients were MYD88MUT, one third of them were CXCR4MUT and 12 to 15 % presented with both mutations.
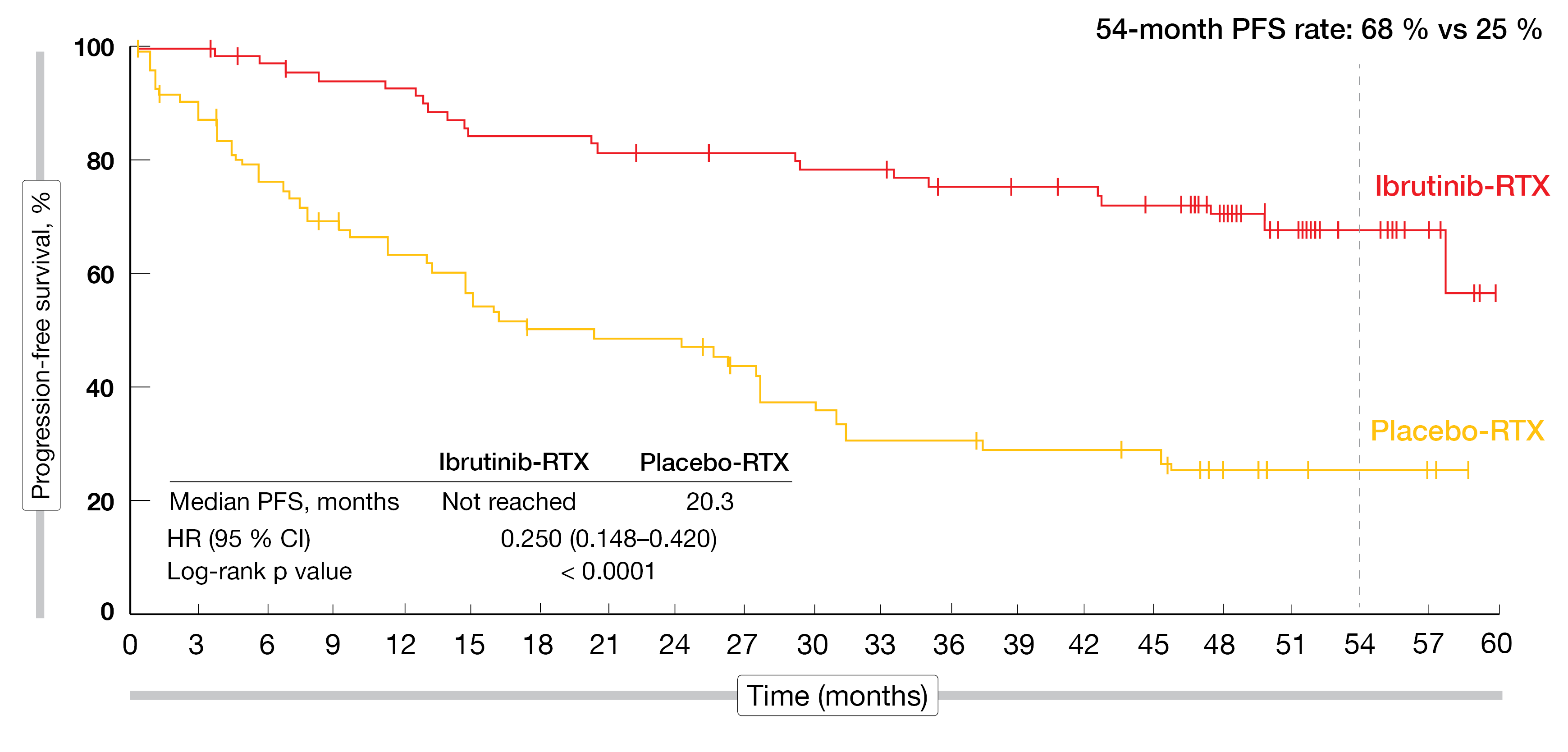
Whereas the median PFS was not reached with five years of treatment (NR vs 20.3 %; HR = 0.250; 95 % CI, 0.148-0.420; p < 0.0001), the 54-month PFS rate demonstrated a strong benefit of ibrutinib plus RTX over placebo plus RTX (68 % vs 25 %, Figure 1). Interestingly, the PFS-benefit of adding ibrutinib to RTX was observed regardless of patients’ genotype and prior treatment status. The major response rate (MRR) was higher in arm A than in arm B (76 % vs 31 %). Although ibrutinib plus rituximab acted quite fast, remission deepened during the study, indicating that some patients were late responders. The higher response rates observed in arm A were independent of genotypes or prior treatment status. Ibrutinib plus RTX was faster than placebo plus RTX in improving and sustaining IgM and Hgb levels. The median OS was not reached in either treatment arm over the five-year follow-up period. There was a substantially higher number of patients with PD in arm B compared to arm A (45 % vs 9 %), as well as more patients receiving a subsequent treatment (63 % vs 12 %, respectively). In total, 47 % of patients in arm B crossed over to single agent ibrutinib after PD.
The prevalence of AEs leading to dose reduction or to treatment discontinuation were quite low (11% in year 3 to 5 % in year 5). Most common treatment-emergent AEs (TEAEs) were arthralgia (13 %), hypertension (10 %) and diarrhea (3 %) in year 4 to 5, whereby the frequency decreased over time (15 %, 17 % and 11 %, respectively, in year 3 to 4).
After a 63-month follow-up, the combination of ibrutinib plus RTX showed its ongoing superiority over placebo plus RTX in patients with WM. Surprisingly, clinical outcomes were independent of patients’ genotype. Over the long-term therapy of ibrutinib plus RTX, a stable and manageable safety profile with no new signals was observed in this patient population.
Figure 1: Progression-free survival rate after five years of ibrutinib plus RTX treatment in the intention to treat population of the INNOVATE study.
Real world data on CIT and BTKi: the WhiMSICAL registry
WhiMSICAL is the first global registry collecting patient-derived data in WM. It aims for a continuously expanding patient-derived dataset, as well as generating hypotheses around WM presentations, treatment, and patient-reported outcomes (PROs) [8].
One of the many gaps in WM clinical research is a head-to-head comparison of BTKis with chemoimmunotherapy (CIT). A recent real-world study demonstrated the relative equivalence of the combination bendamustine plus rituximab (BR) and BTKi in terms of efficacy [9]. In this context, the WhiMSICAL registry was used to identify the treatment efficacy (TTNT) of BTKi versus CIT in real-life conditions, as well as the treatment-related quality of life (QoL). The study presented at iwWM 2022 by Ibrahim Tohidi-Esfahani was initiated and driven by patients of the International Waldenström’s Macroglobulinemia Foundation (IWMF) and affiliates, partnered with international clinicians. Patients were recruited globally through social media [10], completed their consent online (www.cart-wheel.org) and entered information retrospectively and prospectively about their symptoms, pathology, treatment (regimen and date), QoL (EORTC QLQ-C30 score), as well as on COVID-19 infection and vaccines.
After six years, the registry recruited 644 patients from 21 countries. Most patients originated from English speaking countries (USA, 53 %; Australia, 20 %; UK, 9 %). Regarding the first line of treatment, 370 patients were administered CIT (BR, n = 110; rituximab [R], n = 64; dexamethasone/rituximab/cyclophosphamide [DRC], n = 37), while 53 patients received BTKi containing regimens (BTKi monotherapy, n = 42). The BTKi group was significantly older than the CIT group (median age, 66 vs 62 years, p = 0.02) at first treatment and time to 1st treatment was significantly longer (411 vs 82 days, p = 0.009). The median follow-up for the front-line setting was 56 months. With a median follow-up of 38 months, the median TTNT was 87 months with BR; with a median follow-up of 56 months, the median TTNT was 33 months with rituximab; and with a median follow-up of 51 months, the median TTNT was 84 months with DRC and not reached in the BTKi group, with only the rituximab vs BTKi difference being statistically significant.
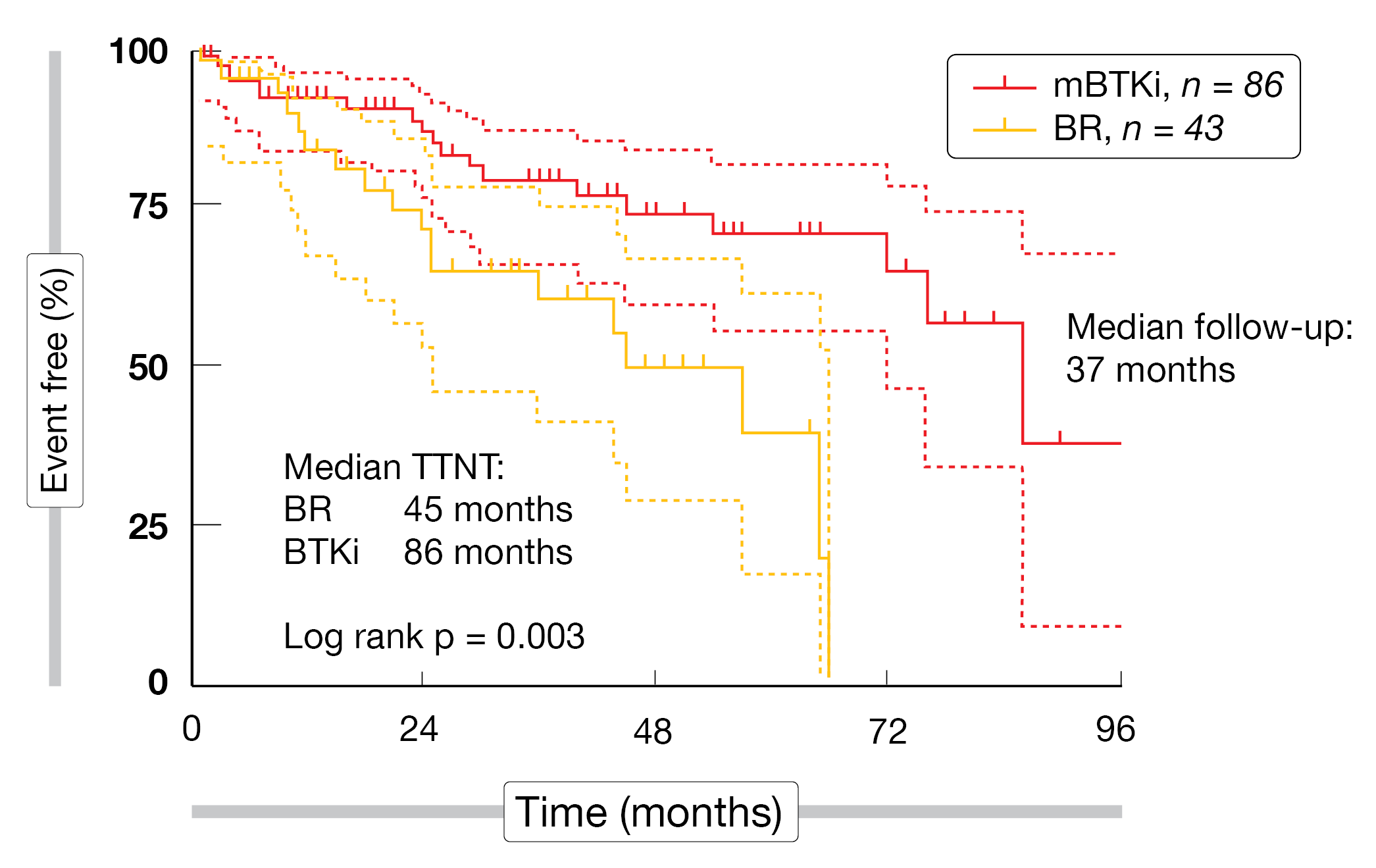
In the relapsed/refractory setting, the most common administered treatments were BTKi monotherapy (n = 86) and BR (n = 43). Patients’ characteristics were similar in terms of age, comorbidities, IgM and Hgb levels between both groups. In total, 40 % of BTKi patients received prior bendamustine treatment, while 9 % of BR patients received a prior BTKi therapy. The median follow-up was 37 months and median TTNT was twice as long in the BTKi group compared to the CIT group (86 months vs 45 months, p = 0.003) (Figure 2). Reported QoL across 1L and R/R setting was significantly higher (p < 0.01) in patients who were still receiving BTKi compared to those who had a BR therapy in the last twelve months.
Real-world data collected from the WhiMSICAL register on CIT versus BTKi treatment demonstrated the superiority of BTKi over BR in terms of TTNT in a relapsed/refractory setting and equivalent TTNT in the 1L. A better QoL was reported by patients treated with BTKi compared to BR. This registry keeps being implemented and will become more and more powerful, giving new insights into WM PROs, as well as facilitating treatment decisions for clinicians and patients.
Figure 2: Time to next treatment (TTNT) of cohorts with bendamustine plus rituximab (BR) vs BTKi therapy in the relapsed/refractory setting.
REFERENCES
- Treon, SP, et al., Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med 2015; 372(15): 1430-1440.
- Castillo, J, et al., Long Term Follow-Up of Ibrutinib Monotherapy in WM. 2022; iwWM 2022, Session 9.
- Castillo, JJ, et al., Long-term follow-up of ibrutinib monotherapy in treatment-naive patients with Waldenstrom macroglobulinemia. Leukemia 2022; 36(2): 532-539.
- Dimopoulos, MA, et al., Phase 3 Trial of Ibrutinib plus Rituximab in Waldenström’s Macroglobulinemia. N Engl J Med 2018; 378(25): 2399-2410.
- Abeykoon, JP, et al., Ibrutinib monotherapy outside of clinical trial setting in Waldenström macroglobulinaemia: practice patterns, toxicities and outcomes. Br J Haematol 2020; 188(3): 394-403.
- Castillo, JJ, et al., Response and Survival Outcomes to Ibrutinib Monotherapy for Patients With Waldenström Macroglobulinemia on and off Clinical Trials. Hemasphere 2020; 4(3): e363.
- Buske, C, et al., Long Term Follow-up of the INNOVATE Study. 2022; iwWM 2022, Session 9.
- Tohidi-Esfahani, I, et al., WhiMSICAL: A global Waldenström’s Macroglobulinemia patient-derived data registry capturing treatment and quality of life outcomes. Am J Hematol 2021; 96(6): e218-e222.
- Abeykoon, J, et al., Bendamustine rituximab (BR) versus ibrutinib (Ibr) as primary therapy for Waldenström macroglobulinemia (WM): An international collaborative study. J Clin Oncol 2022; 40(16): Abstract 7566.
- Tohidi-Esfahani, I, et al., Real World Data on CIT and BTK-inhibitors: WHIMSICAL Study. 2022; iwWM 2022, Session 9.
© 2023 Springer-Verlag GmbH, Impressum
More posts
Emergent BTKi treatments in WM
Emergent BTKi treatments in WM Additionally to ibrutinib, to date the only once-daily B
BTK inhibition in Waldenström’s macroglobulinemia: trial updates and biomarker analysis
BTK inhibition in Waldenström’s macroglobulinemia: trial updates and biomarker analysis
Management of WM patients previously exposed to BTK-inhibitors
Management of WM patients previously exposed to BTK-inhibitors Zanubrutinib in ibrutin
New insights into BTKi treatment of Waldenström‘s macroglobulinemia
New insights into BTKi treatment of Waldenström‘s macroglobulinemia New insights into
Preface – iwWM 2022
Preface – iwWM 2022 © author’s own - Efstathios Kastritis, MD, Plasma Cell Dyscrasia