SSTR-positive neuroendocrine tumors: peptide receptor radionuclide therapy
Real-world insights into re-treatment with the FDA-approved β-emitter 177Lu-DOTATATE
177Lu-DOTATATE was approved by the FDA in 2018 following the encouraging results from the NETTER-1 trial, where a regimen of 4 doses was shown to improve both progression-free survival (PFS) and overall survival (OS) compared to somatostatin analog (SSA) therapy in patients with advanced gastroenteropancreatic neuroendocrine tumors (GEP-NETs) [1]. However, the progression of advanced NETs is inevitable and there is currently a lack of available treatment options for these patients. A retrospective chart review at a single US center evaluated the real-world effectiveness and safety of re-treatment with 177Lu-DOTATATE on progression [2].
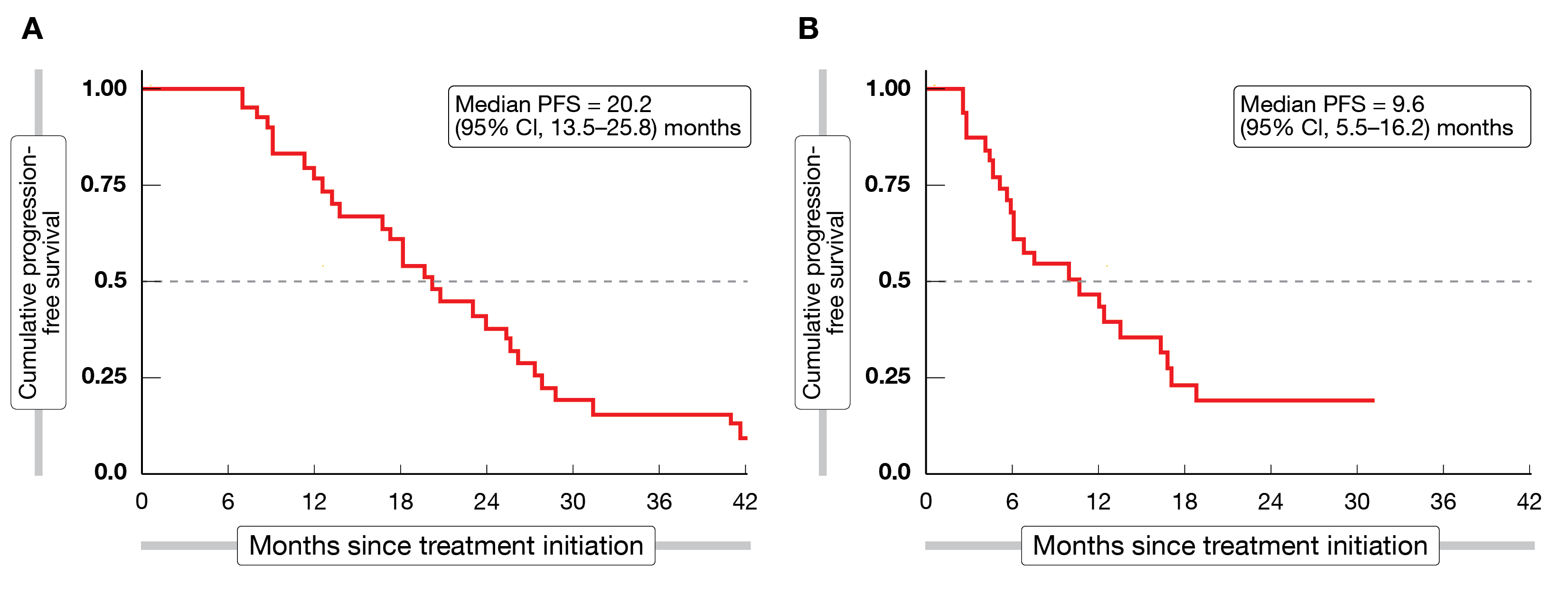
Thirty-one patients with advanced NETs who received initial treatment with up to 4 doses of 177Lu-DOTATATE and who were re-treated with ≥1 additional dose following disease progression and a period of ≥6 months since the end of initial treatment, were evaluated. Patients received a median of 6 doses (4 initial doses and 2 re-treatment doses) and the average administered activity considering all stages of treatment was 41.9 ± 4.4 GBq. Best responses of partial response and stable disease were observed in 11 patients (35 %) and 20 patients (65 %) after initial treatment, and in 7 patients (23 %) and 14 patients (45 %) after re-treatment, respectively. Median progression-free survival (PFS) was 20.2 and 9.6 months after initial and re-treatment (Figure 1), respectively, and median OS was 42.6 months from the start of initial treatment and 12.6 months from the start of re-treatment. Although hematological parameters decreased significantly during both initial and re-treatment, they recovered with no significant difference between the values prior to initial treatment and prior to re-treatment. Only 1 grade 3 hematological adverse event (AE) occurred during initial treatment (neutropenia), while during re-treatment 4 grade 3 AEs were noted (1 leukopenia, 1 anemia, 2 thrombocytopenia). Clinically significant hematotoxicity occurred in 1 and 3 patients following initial and re-treatment, respectively. No grade 3 or 4 nephrotoxicity was observed at any time.
This real-world study provided early evidence supporting re-treatment with 177Lu-DOTATATE, which appeared to be well tolerated and offered disease control in patients with progressive NETs following initial 177Lu-DOTATATE treatment.
Figure 1: Progression-free survival observed from the start of initial treatment (A) and re-treatment with 177Lu-DOTATATE (B)
COMPOSE: 177Lu‑edotreotide, an alternative β-emitter
Well-differentiated aggressive grade 2 and 3 GEP-NETs frequently develop into metastatic disease [4]. The radiolabeled somatostatin analog 177Lu‑edotreotide (177Lu-DOTATOC), a peptide receptor radionuclide treatment (PRRT), has shown potential to expand the treatment landscape for these patients beyond current standard therapies, as it previously demonstrated promising efficacy and a favorable safety profile. In a retrospective study, two or more cycles of 177Lu‑edotreotide had been shown to provide a median PFS of nearly 30 months in metastatic GEP-NET patients [3]. The COMPOSE trial, a randomized, open-label, multicenter, phase III study, was designed to provide prospective data on the efficacy (PFS and OS) and safety of first- or second-line treatment with 177Lu‑edotreotide in patients with SSTR-positive GEP-NETs [5].
Recruitment of patients started in September 2021 and currently includes 29 sites across the globe. At least 202 patients are planned to be randomized 1:1 to either up to six cycles of 177Lu‑edotreotide (7.5 GBq per cycle administered intravenously at 6- to 8-week intervals) or an active comparator (capecitabine and temozolomide (CAPTEM) or folinic acid + fluorouracil + oxaliplatin (FOLFOX) chemotherapy, or everolimus according to investigator’s choice). Interestingly, the authors noted that results from the control arm would provide prospective data on the efficacy of CAPTEM chemotherapy in grade 2 and 3 GEP-NETs, which is currently lacking too.
Expanding the range of PRRT options: promising results with α-emitters
PRRT with the β-particle emitter 177Lu-DOTATATE is currently considered the standard of care (SoC) for patients with SSTR-positive GEP-NETs [6]. Despite the demonstrated benefits of 177Lu-DOTATATE, there is a growing interest in α-emitter therapy with isotopes such as 212Pb and 225Ac, which have higher linear energy transfer (80–100 keV/μm) and a shorter path length (40-100 μm) than β-emitters. As such, they have the potential to improve both the efficacy and safety of PRRT by causing irreversible DNA damage (i.e., double-strand breaks) in cancer cells as well as less collateral damage in healthy tissues, which should reduce the toxicity of the treatment [7].
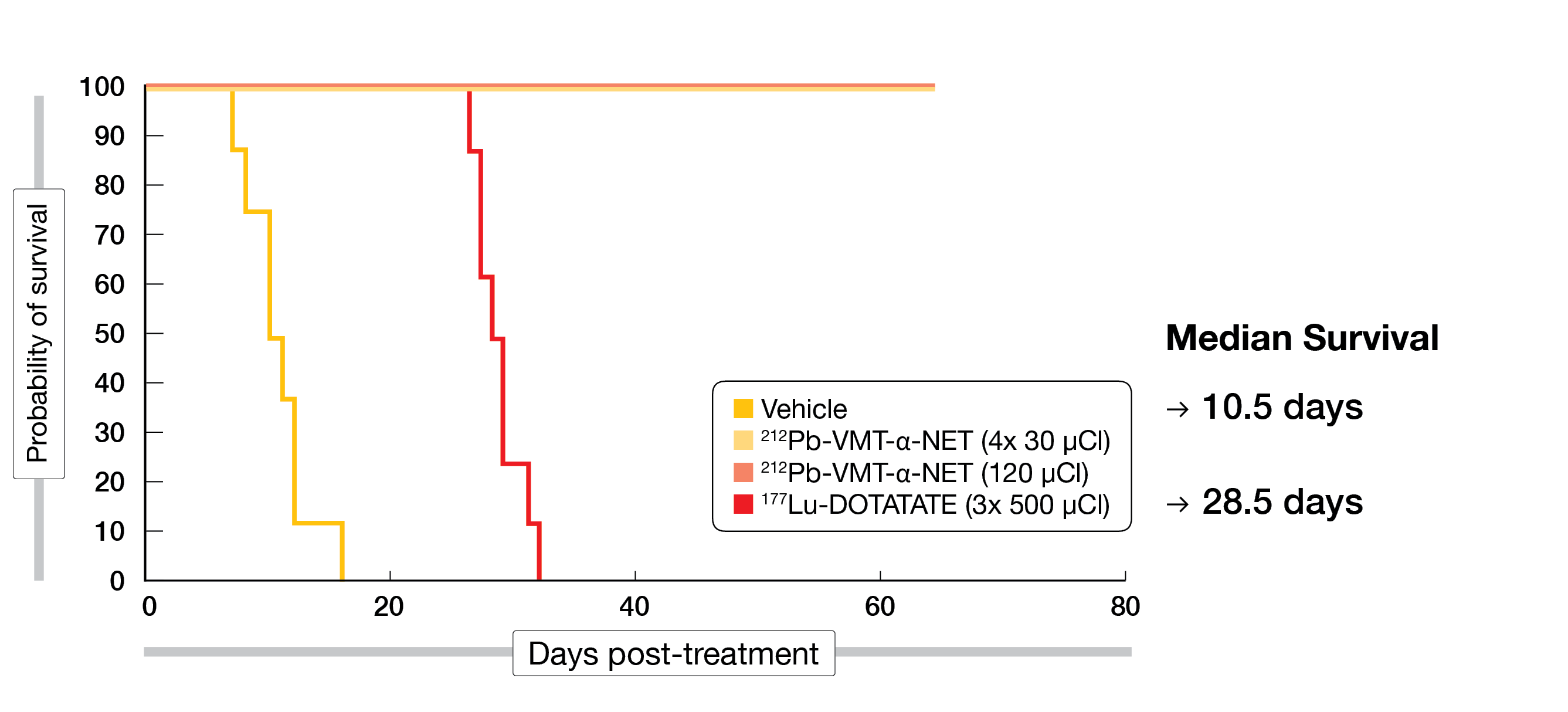
In a preclinical study presented by Schultz et al., the efficacy of the novel α-emitter 212Pb-PSC-PEG2-TOC was evaluated and compared to 177Lu-DOTATATE in a mouse model. Single or fractionated doses of 212Pb-PSC-PEG2-TOC (total activity at 4.44 MBq) were intravenously injected in tumor-bearing mice and 100% complete tumor responses were achieved (as of day 65 post-therapy initiation). 212Pb-PSC-PEG2-TOC was well tolerated in comparison with 177Lu-DOTATATE, which resulted in an improved OS compared to the vehicle cohort (28.5 days vs. 10.5 days) but no complete responses were observed (Figure 2) [8]. The promising efficacy of 212Pb-PSC-PEG2-TOC demonstrated in this preclinical setting warrants further investigation in SSTR-positive NETs.
In line with these data, preliminary results from a phase I dose-escalation trial evaluating the α-emitter 212Pb-DOTAMTATE in SSTR-positive NET patients were also presented at NANETS 2022. Metastatic NET patients with histologically confirmed tumors from any primary site were included in the study and treated with the recommended phase 2 dose (RP2D) of 4 cycles of 2.50 MBq/kg administered intravenously at 8-week intervals. An objective radiological response was observed in 10 out of 12 PRRT-naïve patients, while 6 out of 10 patients who had progressed after prior PRRT therapy with 177Lu-DOTATATE demonstrated an objective response (ORR 60 %). The treatment was well tolerated in all patients, with the most common treatment-emergent adverse events (TEAEs) being nausea, fatigue and alopecia. There were no serious drug-related TEAEs, and no dose reductions or treatment delays were required [9].
The authors concluded that 212Pb-DOTAMTATE demonstrated promising efficacy and a favorable safety profile. Enrolment in a phase II trial aiming to confirm the safety and efficacy of 212Pb-DOTAMTATE in a larger cohort of patients with advanced NETs was ongoing at the time of presentation.
Figure 2: Overall survival benefit with 212Pb-PSC-PEG2-TOC compared to 177Lu-DOTATATE in a preclinical mouse model
ACTION-1: α-emitter 225Ac-DOTATATE after progression on 177Lu-based PRRT
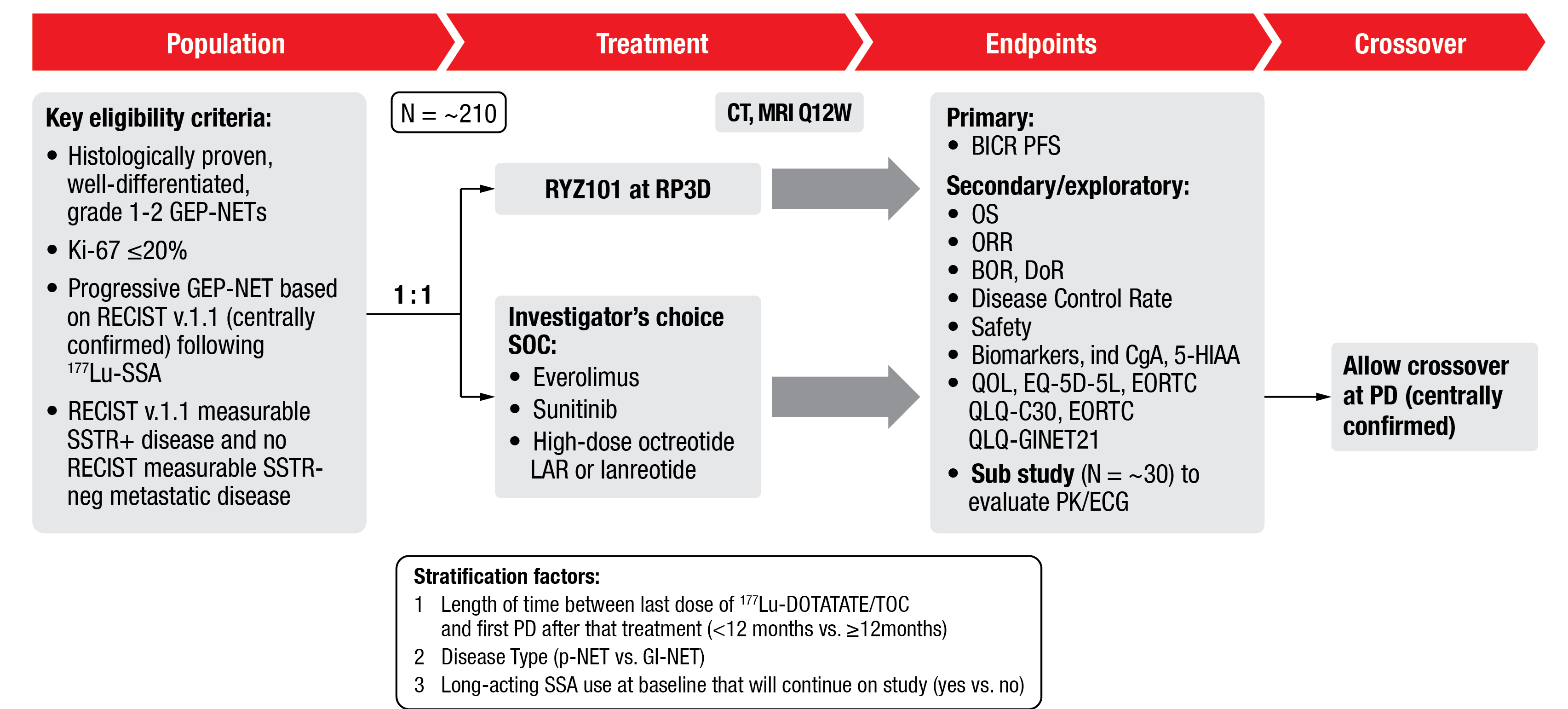
225Ac-DOTATATE (RYZ101), another α-emitter, is currently being investigated for the treatment of SSTR-positive well-differentiated GEP-NETs. The ACTION-1 study is a randomized, open-label phase 1b/3 trial designed to first determine the safety, pharmacokinetics and recommended phase 3 dose (RP3D) of 225Ac-DOTATATE (Phase 1b). In the second part of this study (Phase 3), its efficacy at the RP3D compared to the SoC (everolimus, sunitinib, or high-dose long-acting SSAs) will be assessed in patients with advanced GEP-NETs who have progressed following 2-4 cycles of prior PRRT with 177Lu-labeled SSAs.
Enrolment in ACTION-1 part 1 is ongoing and currently includes about 6 sites across the US. The starting dose planned for this study is 120 kBq/kg administered intravenously every 8 weeks for up to 4 cycles, which will be de-escalated if necessary. Upon end of part 1, ~210 patients will be recruited at around 60 international sites and randomized 1:1 to receive either 225Ac-DOTATATE at the previously established RP3D or investigator’s choice of SoC. The primary endpoint of this study will be PFS by RECIST v1.1 (Figure 3) [10].
Figure 3: Study design of the part 2 (Phase 3) of the ACTION-1 trial
Exploring combination treatments to enhance PRRT
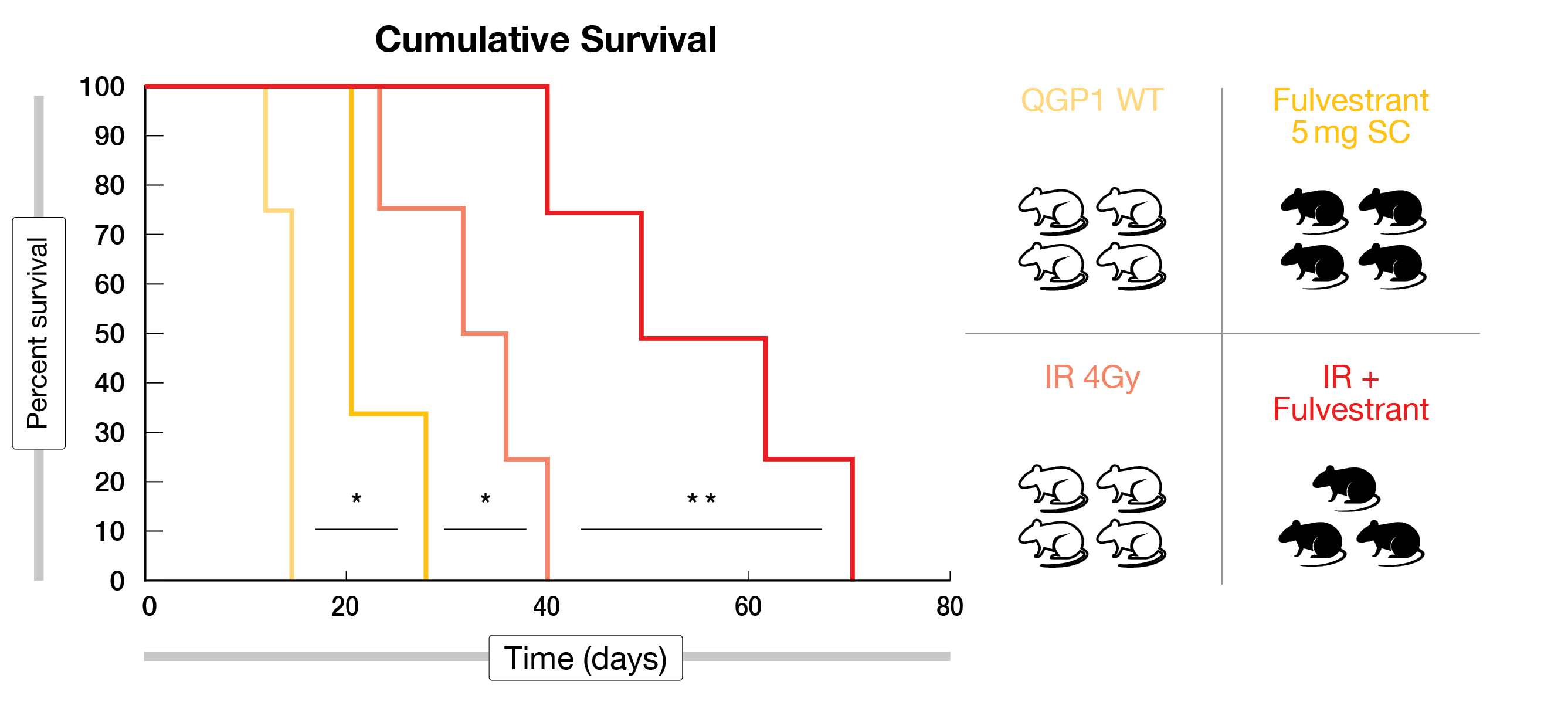
18-30 % of patients do not respond to 177Lu-DOTATATE [1]. Upgrading of the tumor and loss of SSTR expression through diverse epigenetic mechanisms, as well as the increased activity of DNA repair pathways that prevent radiation from inducing DNA damage, have been proposed as potential causes of PRRT refractory disease [11]. Several preclinical studies presented at NANETS 2022 evaluated the combination of PRRT with other targeted therapies to improve its efficacy. On the one hand, two studies have shown that SSTR2 expression can be increased in NET cells by targeting various epigenetic enzymes that negatively control its expression, including DNA-methyltransferase (DNMT) and histone deacetylases (HDACs) [12, 13]. In line with this, enhanced uptake of 68Ga-DOTATATE was observed in NET cells treated with VPA (HDAC inhibitor) and decitabine (DNMT inhibitor), as compared with either single drug [13]. Moreover, both fulvestrant (ESR1 inhibitor) and ATRA (Pin1 inhibitor) have been shown to radiosensitize NET cells by significantly decreasing expression of DNA repair genes such as BRCA1 and RAD51, and to delay tumor growth and extend survival in a mouse xenograft model when combined with radiation (Figure 4) [14, 15].
Figure 4: Cumulative survival results for Fulvestrant plus IR compared to Fulvestrant or IR alone in QGP1 tumor-bearing mice
CALR: a novel target for PRRT in pNETs
Current theranostic techniques have taken advantage of the common overexpression of SSTR2 in well-differentiated GEP-NETs by targeting them with radiolabeled SSAs. However, approximately 25 % of low-grade, as well as most high-grade, pancreatic NETs (pNETs) are reported to lack SSTR expression and thus will not benefit from available therapies. Thus, alternative targets are required to provide new treatment options for these patients [16].
Preliminary results from a preclinical study presented at NANETS 2022 highlighted the potential of calreticulin (CALR) as an alternative diagnostic and therapeutic target for pNET patients with low expression of SSTRs. CALR is usually located in the endoplasmic reticulum and can transiently translocate to the cell membrane in response to certain stimuli [17]. In this study, surface translocation of CALR was induced in pNET cells to be detected by a novel radiolabeled peptide (68Ga-CALR). In mice, 68Ga-CALR was shown to be rapidly cleared via the kidneys and no significant uptake was seen in vital organs when injected at 3 MBq [18]. The authors concluded that these data strongly support the potential of CALR-radiolabeled peptides as new tools for the diagnosis and treatment of a subset of pNET patients.
REFERENCES
- Strosberg J et al., Phase 3 trial of 177Lu-DOTATATE for midgut neuroendocrine tumors.
N Engl J Med 2017; 376(2): 125-135 - Delpassand ES et al., Effectiveness and safety of re-treatment with 177Lu-DOTATATE in patients with progressive NETs in the US: a retrospective real-world study. NANETS 2022, abstract C-15
- Baum RP et al., [(177)Lu-DOTA](0)-D-Phe(1)-Tyr(3)-octreotide ((177)Lu-DOTATOC) for peptide receptor radiotherapy in patients with advanced neuroendocrine tumours: a Phase-II study. Theranostics 2016; 6(4): 501-10
- Rindi G et al., A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol 2018; 31(12): 1770-1786
- Halfdanarson TR et al., COMPOSE: pivotal Phase III trial for well-differentiated aggressive grade 2/3 gastroenteropancreatic neuroendocrine tumors comparing 177Lu-edotreotide with best standard of care. NANETS 2022, abstract T-2
- Hope TA et al., NANETS/SNMMI Procedure Standard for Somatostatin Receptor-Based Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE. J Nucl Med 2019; 60(7): 937-943.
- Koh TT et al., Targeted alpha-particle therapy in neuroendocrine neoplasms: A systematic review. World J Nucl Med 2021; 20(4): 329-335
- Schultz MK et al., [212Pb]PSC-PEG2-TOC therapy for NET leads to complete responses in mice bearing SSTR2 positive tumors – comparison to [177Lu]DOTATATE in a preclinical model. NANETS 2022, abstract B-16
- Delpassand ES et al., Targeted α-Emitter therapy with 212Pb-DOTAMTATE for the treatment of metastatic SSTR-expressing neuroendocrine tumors: first-in-humans dose-escalation clinical trial. J Nucl Med 2022; 63(9): 1326-1333
- Hope T et al., ACTION-1: a randomized Phase Ib/3 trial of RYZ101 compared with SoC in SSTR2+ well-differentiated GEP-NET with progression following Lu-177 SSA. NANETS 2022, abstract T-6
- Adant S et al., Combination treatments to enhance peptide receptor radionuclide therapy of neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2020; 47(4): 907-921
- Madigan JP et al., Multiple layers of epigenetic regulation cooperate to silence expression of somatostatin receptor type 2 in pancreatic neuroendocrine tumors. NANETS 2022, abstract B-2
- Whitt J et al., Simultaneous inhibition of DNA methylation and histone de-acetylation for enhanced SSTR2 expression in vitro. NANETS 2022, abstract B-4
- Schwarz JL et al., Inhibition of estrogen receptor alpha radiosensitizes neuroendocrine tumors. NANETS 2022, abstract B-10
- Williams JK et al., All-trans retinoic acid radiosensitizes neuroendocrine tumor cells via peptidyl-prolyl cist-trans isomerase 1 inhibition. NANETS 2022, abstract B-7
- Mehta S et al., Somatostatin Receptor SSTR-2a Expression Is a Stronger Predictor for Survival Than Ki-67 in Pancreatic Neuroendocrine Tumors. Medicine (Baltimore) 2015; 94(40): e1281.
- Obeid M et al., Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev 2007; 220: 22-34
- Guenter R et al., Detecting cell surface expression of calreticulin in pancreatic neuroendocrine tumors using a novel [68Ga]-radiolabeled peptide. NANETS 2022, abstract B-6
© 2023 Springer-Verlag GmbH, Impressum
More posts
Preface – NANETS 2022
Preface – NANETS 2022 © private - Mauro Cives, MD, Department of Interdisciplinary Me