Challenges and State of the Art: The advanced stage patient
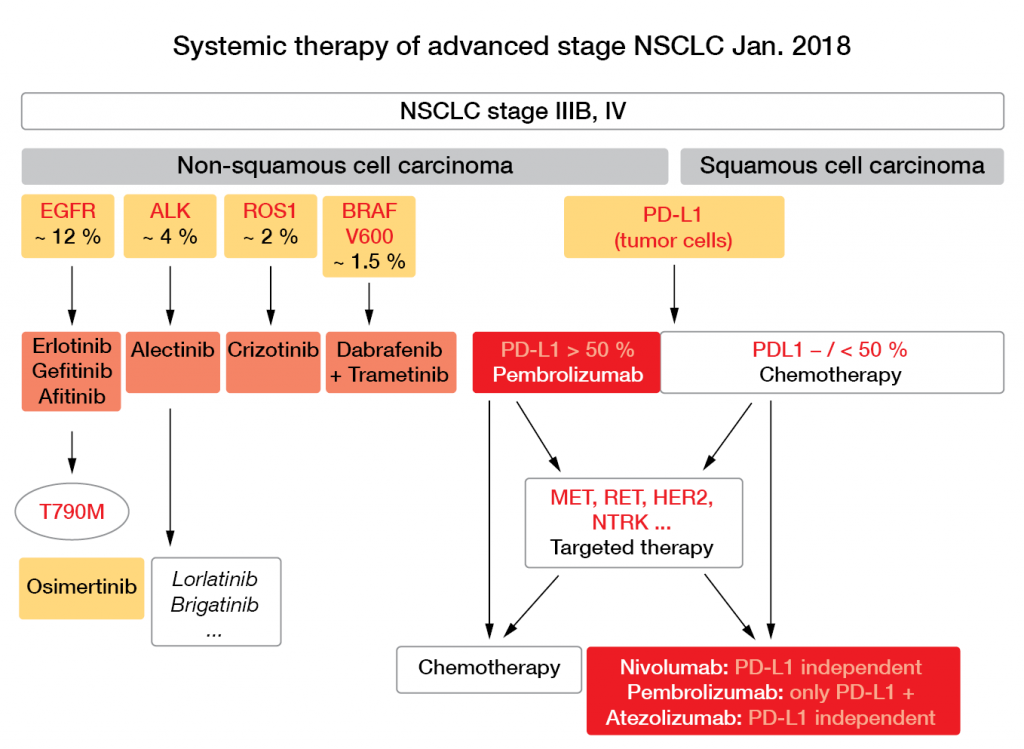
With 2-year OS of < 15 %, the prognosis of patients with advanced lung cancer has been invariably poor for decades, irrespective of the chemotherapy that was combined with platinum compounds, as Jürgen Wolf, MD, Department of Internal Medicine I, University Hospital of Cologne, remarked (e. g. [1]). Consequently, as Scheffler pointed out, in recent years there has been a dramatic decline in published trials using chemotherapy alone, and in contrast, a steep rise in the numbers of trials with targeted and immune therapies. This is also reflected in the current recommendations proposed by Wolf for systemic therapy of patients in advanced stages of NSCLC (Fig. 4).
Figure 4: Systemic therapy of advanced stage NSCLC as of January 2018. Courtesy of J. Wolf.
Targeted therapies: Tyrosine kinase inhibitors
When targeted therapies were first tried for NSCLC they showed only marginal activities in unselected patients and for advanced stages. The first real breakthrough in systemic therapy was promoted by the identification of driver mutations in certain genes; e. g. the genes for epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS1, and BRAF, among others. The detection of these mutations allowed the selection of patients whose tumors were sensitive to the specific tyrosine kinase inhibitors (TKIs) of the mutated proteins. With this type of directed, or personalized, therapy, rapid progress has been made possible over the last few years [2].
At present, three EGFR TKIs are approved in the European Union for first-line therapy of EGFR-mutant NSCLC: gefitinib, erlotinib, and the second-generation afatinib. These have all proven to be superior to chemotherapy in terms of responses and PFS. However, as Richard Riedel, MD, Department of Internal Medicine I, University Hospital of Cologne, pointed out, development of resistance to these drugs is almost inevitable. While in cases of limited progression (e. g., in the CNS, or in single extra-CNS sites) continuation of the first-line drug might be an option, probably in conjunction with local therapeutic approaches, systemic progression in multiple sites invariably calls for a change in systemic therapy. To decide on the follow-up therapy, investigation of the mechanism of resistance is obligatory.
In more than half of the cases, resistance against first-line EGFR inhibitors is caused by the T790M mutation of the EGFR gene. Tumors with this mutation can be successfully treated with the third-generation TKI osimertinib: in the phase III AURA 3 trial, osimertinib more than doubled PFS in these patients, compared to platinum-pemetrexed chemotherapy (median PFS, 10.2 vs. 4.4 months; HR, 0.30; p < 0.001; [3]). Furthermore, in the first-line phase III FLAURA trial, osimertinib was superior to standard of care in unselected patients with newly diagnosed, advanced, EGFR-mutated NSCLC (median PFS, 18.9 vs. 10.2 months; HR, 0.46; p < 0.0001; [4]). Approval of osimertinib for this indication is currently pending.
In about 10 % of cases, resistance against first- or second-generation TKIs is associated with cMET amplification. In these patients, combinations of first- or third-generation TKIs with new agents, like savolitinib, appear to be beneficial in terms of responses [5, 6]; however, at the moment, none of these new agents have been approved.
For patients with NSCLC and ALK translocations, the second-generation ALK inhibitor alectinib has been approved for first-line therapy on the basis of phase III data that showed its superiority over crizotinib, with PFS of about 26 months. When progressing, these tumors must be re-biopsied to identify the mechanism of resistance, which determines the further lines of treatment.
Immunotherapy
A second paradigm shift in the treatment of advanced NSCLC was made possible some years ago by the deepening of our understanding of how immune tolerance of T-lymphocytes against cancer cells is mediated. Immune checkpoint inhibitors such as anti-PD/PD-L1 or anti-CTLA-4 monoclonal antibodies can unleash an anti-tumor immune response by interfering in the T-cell priming and effector phases. By introducing this new type of immunotherapy, a new avenue of cancer therapy has been opened up for a wide variety of cancers, including lung cancer, as Diana S.Y. Abdulla, MD, Department of Internal Medicine I, University Hospital of Cologne, explained.
At present, there are data from a series of phase III trials that show superior OS with PD-1/PD-L1 immune checkpoint inhibition versus chemotherapy in patients with pretreated NSCLC, irrespective of the histological subtype (i.e., squamous or non-squamous NSCLC). The corresponding studies are CheckMate-017/-057 (nivolumab; [7]), KEYNOTE-010 (pembrolizumab; [8]) and the OAK trial (atezolizumab; [9]).
For first-line therapy, only one checkpoint inhibitor is available at present: pembrolizumab has been approved for patients with newly diagnosed stage IV NSCLC with PD-L1 expression of ≥ 50 % (given as the tumor proportion score), which was on the basis of the results of the KEYNOTE-024 trial [10]. In this phase III trial, pembrolizumab resulted in significant prolongation of PFS compared to platinum-based chemotherapy (median PFS, 10.3 vs. 6.0 months; HR 0.50; p < 0.001), as well as improved OS (median OS, 30.0 vs. 14.2 months; HR 0.63; p = 0.002) [11].
Thus, within a little over a decade, the therapeutic landscape of advanced NSCLC has changed dramatically by the introduction of various new drugs with distinct mechanisms of action. In 2018, targeted therapy has been approved for four driver mutations in advanced NSCLC in the first-line setting. To enable these patients to benefit from such treatments, it is indispensable for all patients with a first diagnosis of an adenocarcinoma to be tested for mutations in their EGFR, ALK, ROS1 and BRAF genes. Immune checkpoint inhibitors that target PD-1 are indicated for first-line treatment when EGFR and ALK are wild-type and ≥ 50 % of the carcinoma cells express the PD-L1 protein in the histology-based test, defining their ‘PD-L1 immunohistochemistry’ (IHC). Thus, both sequencing and IHC are necessary to guide treatment decisions here. In the second-line setting, checkpoint inhibitors can be used independent of the PD-L1 and mutational status.
In cases of relapse after TKI therapy, identification of the resistance mutations is essential to define the treatment with the next generation inhibitors, to overcome the mechanism(s) of resistance.
Angiogenesis
Among the ‘hallmarks of cancer’ postulated by Hanahan and Weinberg in 2000 [12], the induction of angiogenesis is among those that can already be approached therapeutically. Attempts to interfere with tumor-induced neo-angiogenesis (e. g., by blocking the VEGF, FGF or PDGF pathways) have met with some, although heretofore limited, success. For example, nintedanib is a triple angiokinase inhibitor as it can block all three of the above-mentioned mechanisms, and in the phase III LUME-Lung 1 trial it led to prolongation of OS in patients with adenocarcinoma histology, from a median of 10.3 to 12.6 months (HR, 0.83; p = 0.0359). When the analysis was restricted to the predefined population of patients with adenocarcinoma who had progressed within 9 months of the start of first-line therapy, OS was also significantly longer in the docetaxel plus nintedanib group compared to the docetaxel plus placebo group (median OS, 10.9 vs. 7.9 months; HR, 0.75; p = 0.0073; Figs. 4, 5; [13]). To improve upon results like these, biomarkers for selection of patients would be helpful, but these are currently lacking for anti-angiogenic therapies. Another promising approach might be a combination of strategies, as Scheffler stated; for instance, by normalizing tumor vasculature up-front with the aid of angiogenesis inhibitors, and subsequently adding other therapies with different modes of action.
Figure 5: The LUME-Lung 1 trial. Overall survival of patients with adenocarcinoma histology and time since start of first-line therapy of less than 9 months, as docetaxel plus nintedanib versus docetaxel plus placebo. Modified from [10].
REFERENCES
- Schiller et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002; 346: 92-8.
- The Clinical Lung Cancer Genome Project and Network Genomic Medicine. A genomics-based classification of human lung tumors. Sci Transl Med 2013; 5: 209ra153.
- Mok T et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017; 376: 629-40.
- Ramalingam S et al. Osimertinib versus standard of care (SoC) EGFR-TKI as first-line therapy in patients (pts) with EGFRm advanced NSCLC: FLAURA. ESMO 2017, Abstract #LBA2_PR.
- Yang J et al. A phase Ib trial of savolitinib plus gefitinib for Chinese patients with EGFR-mutant MET-amplified advanced NSCLC. J Thorac Oncol 2017; 12 (Suppl): 120 (WCLC 2017; Abstract #OA 09.06).
- Ahn M et al. TATTON Phase Ib expansion cohort: osimertinib plus savolitinib for pts with EGFR-mutant MET-amplified NSCLC after progression on prior EGFR-TKI. J Thorac Oncol 2017; 12 (Suppl): 120 (WCLC 2017, Abstract #OA 09.03).
- Barlesi et al. Long-term outcomes with nivolumab versus docetaxel in patients with advanced NSCLC: CheckMate 017 and CheckMate 057 2-y update. ESMO 2016, Abstract #1215PD.
- Herbst RS et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2015; 387: 1540-50.
- Barlesi F et al. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC. ESMO 2016, Abstract #LBA44_PR
- Reck M et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823-33.
- Brahmer JR et al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced NSCLC with PD-L1 TPS ≥50 %. J Thorac Oncol 2017; 12 (Suppl): 137 (WCLC 2017, Abstract #OA 17.06).
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57-70.
- Reck M et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014; 15: 143-55.
- Reck M et al. KEYNOTE-024: Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced NSCLC with a PD-L1 tumour proportion score (TPS) >50 %. ESMO 2016, Abstract #LBA8_PR.
- Cousin S et al. Toxicity profiles of immunotherapy. Pharmacol Therapeut 2018; 181: 91-100.



![Figure 5: The LUME-Lung 1 trial. Overall survival of patients with adenocarcinoma histology and time since start of first-line therapy of less than 9 months, as docetaxel plus nintedanib versus docetaxel plus placebo. Modified from [10].](https://memoinoncology.com/wp-content/uploads/2020/04/Grafik-7-preceptorship-cologne-en.png)


