Screening and early detection of lung cancer
According to the principles stated by the World Health Organisation, screening programmes are aimed at timely detection of diseases that represent important health problems and for which there are accepted treatments [1]. Suitable and acceptable tests are required, and the cost of case finding should be economically balanced. “Lung cancer is definitively a relevant health problem, which is why we need early detection,” stated Rudolf Maria Huber, MD, Division of Respiratory Medicine and Thoracic Oncology, Thoracic Oncology Centre Munich, University of Munich, Germany. The incidence rates are high, and 60 % of patients die within one year of diagnosis. Symptomatic lung cancer is almost always detected at an advanced stage, where cure is hardly ever achieved.
From X-ray to low-dose computed tomography
Lung-cancer screening in the 1970s, 1980s and 1990s involved chest radiography and sputum cytology. Ten prospective X-ray trials with and without cytology were conducted between 1951 and 1985, but none of these demonstrated any clinically relevant benefits of screening. For instance, the randomised, controlled Mayo Lung Project showed that more lung-cancer cases were detected in the screening group than in the control group, but the lung-cancer death rates did not differ between these two groups [2]. Based on the available evidence, almost all of the scientific societies and legal authorities recommend against screening by chest X-rays or sputum cytology.
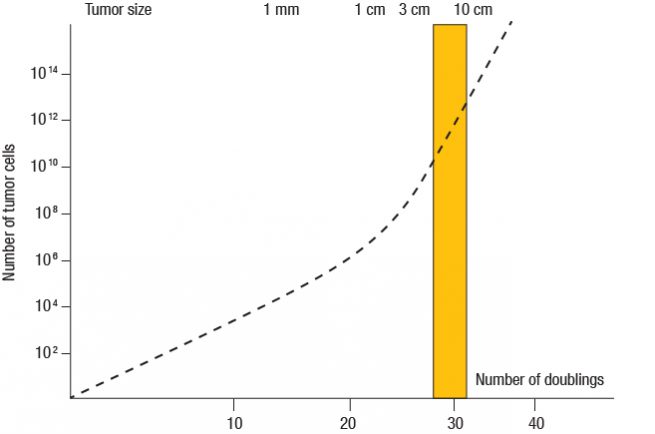
The lack of benefit of screening using chest X-rays can be due to the advanced size of nodules at the time of their routine clinical detection, with the tumour cells having already gone through many doubling times. “The diameter of these lesions is usually 2 cm to 10 cm,” noted Dr. Huber (Figure 1). Computed tomography (CT), on the other hand, enables identification of nodules of 4 mm, or even less. “This technique is much more sensitive than chest X-rays.”
Figure 1: Typical tumour size at the time of routine clinical detection with X-rays (yellow box)
The National Lung Screening Trial
Many prospective studies have investigated lung-cancer screening using low-dose CT. The large randomised American National Lung Screening Trial (NLST) included 53,464 high-risk subjects, and it yielded positive results. People aged 55 to 74 years who had a smoking history of 30 or more pack years were randomised to CT or chest X-rays. Former smokers had quit smoking within the previous 15 years. The participants underwent three annual screens. “As compared to radiography, low-dose CT screening promoted a relative reduction in lung-cancer mortality of 20 %,” Dr. Huber reported (Figure 2) [3]. “Overall mortality was reduced by 6.7 %.” As in the Mayo Project, the cumulative number of tumours detected by CT exceeded the number found in the control arm, but the patients benefited from it to a clinically relevant extent.
From a statistical point of view, the positive predictive value estimated in the NLST was higher for chest X-rays than for low-dose CT (5.7 % vs. 3.8 %), although X-rays were clearly less sensitive (74 % vs. 94 %) [4]. “On the other hand, a normal chest CT scan does not guarantee the absence of cancer,” Dr. Huber pointed out. Interval cancers (tumours that emerged in-between screenings) occurred in 26 people participating in the NLST, and 42 % of these were stage IV [5]. Endobronchial tumours do not regularly show on CT. “ Patients undergoing a screening programme have to be informed that there is the possibility of missed cases.” Also, radiation exposure confers a slightly increased risk of leukaemia and brain cancer [6].
Potential confounders
Based on the NLST, researchers have estimated that 320 people need to be screened with low-dose CT to prevent one death [3]. Expenses for one life year gained were estimated at US$ 52,000 [7]. For one quality-adjusted life year gained, this was US$ 81,000 [7]. “These results depend one many assumptions, and accordingly vary widely across subgroups, and they have to be calculated differently for every country,” Dr. Huber said.
Moreover, bias is prone to interfere with the interpretation of trials, such as lead-time bias, length-time bias, and over-diagnosis bias. For the NLST, there was also participation bias, as participants were younger and better educated than those who usually develop lung cancer [8]. Also, they were less likely to be current smokers. European studies such as the Danish Lung-Cancer Screening Trial [9] and the DANTE trial [10] prospectively assessed CT-based lung-cancer screening, with negative results so far. Dr. Huber cautioned against a meta-analysis due to differences in design.
Due to these mixed trial results, recommendations on the use of lung-cancer screening vary between countries. The American College of Chest Physicians and the American Society of Clinical Oncology recommend annual screening with low-dose CT for smokers and former smokers (of ≥ 30 pack years) aged 55 to 74 [11]. A joint statement of the German Respiratory and Radiological Societies says that native low-dose CT can be justified on an individual basis (as individual early detection) [12]. Intensive patient education is necessary, which includes smoking cessation advice, as well as interdisciplinary management and quality assurance. The French National Authority for Health commissioned experts to carry out a systematic review on the effectiveness, acceptability and safety of lung-cancer screening with low-dose CT in subjects highly exposed to tobacco [13]. “They concluded that screening should not be recommended in this population,” Dr. Huber reported.
How to improve early detection
“We all agree that screening needs to be improved,” Dr. Huber stressed. This particularly applies to the definition of risk populations and the work-up, with the aim being to avoid false-positive findings. General screening of the US population using the NLST criteria would detect 26.7 % of all lung cancers [14], but reimbursement represents an unsolved issue in this context. Another problem relating to screening programmes is the lack of tools for the assessment of never or light smokers.
With regard to the definition of risk populations, at the ASCO Congress, Aberle et al. presented eligibility criteria for population screening [15]. These are now being introduced in the US. “NLST-based risk factor scoring shows that the preventive effect is greatest in people with the highest risk,” said Dr. Huber [16]. However, models involving multiple variables are not the only tests that make risk stratification possible. Lung function testing, which is simple and inexpensive, allows for very good discrimination. “The lung-cancer risk rises exponentially in men if FEV1 is reduced”, Dr. Huber emphasised [17] (Figure 3). Likewise, decreased carbon monoxide diffusion capacity indicates increased risk, which is true for both sexes [18]. A more sophisticated tool is exhaled breath analysis that differentiates between benign and malignant nodules [19]. Moreover, a blood-based proteomic classifier score was shown to characterise pulmonary nodules as either benign or malignant at the molecular level with high confidence [20]. All of these tools can contribute to the improvement of lung-cancer diagnosis and can avoid unnecessary invasive procedures.
False positives and over-diagnosis
False-positive findings that give rise to pointless diagnostic and therapeutic measures are of great concern in the setting of lung-cancer screening. According to data from the NLST, 30 % of all surgical procedures were performed in patients with benign disease [4]. “This happened even though experienced centres were involved that routinely used CT-guided biopsy,” Dr. Huber noted. Another important aspect relates to over-diagnosis. More than 18 % of all lung cancers detected by low-dose CT in the NLST appeared to be indolent [21]. The probability of over-diagnosis in a bronchioalveolar lung-cancer case detected by low-dose CT was 78.9 %. Statistically, for one patient who stayed alive due to screening in the NLST, 1.38 patients were over-diagnosed. As Dr. Huber pointed out, this needs to be kept in mind when implementation of screening is discussed.
The Lung CT Screening Reporting and Data System (Lung-RADS™), which was designed to standardise lung-cancer screening CT reporting and management recommendations, decreases the probability of false positives. Also, volumetric measurements of nodules help to decide whether invasive diagnosis should be used [22]. Online calculators can facilitate the assessment of the probability of malignancy in a given nodule (www.brocku.ca/cancerpredictionresearch).
Is prevention better than detection?
he NLST-based analysis by Tanner et al. suggested that smoking cessation is just as effective as early detection of lung cancer [23]. “Former smokers in the control arm who had abstained for seven years showed a 20 % mortality reduction,” Dr. Huber noted. Compared to this group, current smokers showed increased lung-cancer–specific mortality and all-cause mortality, irrespective of screening arm. The maximum benefit occurred in patients who received CT screening and who had abstained from smoking for at least 15 years; here, a 38 % reduction in lung-cancer–specific mortality was seen.
These observations were even surpassed by an Italian trial that compared current smokers with ex-smokers [24]. “The benefit of smoking cessation appeared to be 3-fold to 5-fold greater than that achieved by early detection in the NLST trial.” The survival curves separated throughout the follow-up period. “I believe that we have to focus on prevention,” Dr. Huber summarised. “Nevertheless, we should try to improve detection techniques and algorithms.”
REFERENCES
- Wilson JMG & Junger G, Principles and Practice of Screening for Disease. WHO. Geneva, Switzerland 1968
- Fontana RS et al., Screening for lung cancer. A critique of the Mayo Lung Project. Cancer 1991; 67: 1155-1164
- National Lung Screening Trial Research Team, Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365(5): 395-409
- National Lung Screening Trial Research Team, Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013; 368(21): 1980-1991
- Aberle DR et al., Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013; 369: 920-931
- Pearce MS et al., Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 2012; 380: 499-505
- Black WC et al., Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med 2014; 371: 1793-1802
- National Lung Screening Trial Research Team, Baseline characteristics of participants in the randomized national lung screening trial. Natl Cancer Inst 2010; 102: 1771–1779
- Wille MM et al., Results of the randomized Danish Lung Cancer Screening Trial with focus on high-risk profiling. Am J Respir Crit Care Med 2016; 193: 542-551
- Infante M et al., Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med 2015; 191(10): 1166-1175
- American Society of Clinical Oncology 2011
- Vogelmeier C et al., Joint statement of the German Respiratory Society and the German Roentgenological Society on the early detection of lung cancer by low-dose CT. Pneumologie 2011; 65: 5-6
- Coureau G et al., Low-dose computed tomography screening for lung cancer in populations highly exposed to tobacco: A systematic methodological appraisal of published randomised controlled trials. Eur J Cancer 2016; 61: 146-156
- Pinsky PF & Berg CD, Applying the National Lung Screening Trial eligibility criteria to the US population: what percent of the population and of incident lung cancers would be covered?
J Med Screen 2012; 19: 154-156 - Aberle DR, Lecture “Lung Cancer Screening”, ASCO Congress 2016
- Kovalchik SA et al., Targeting of low-dose CT screening according to the risk of lung-cancer death. Engl J Med 2013; 369: 245-254
- Tammemagi MC et al., Incremental value of pulmonary function and sputum DNA image cytometry in lung cancer risk prediction. Cancer Prev Res; 2011; 4(4): 552-561
- de Torres JP et al., Identification of COPD patients at high risk for lung cancer mortality using the COPD-LUCSS-DLCO. Chest 2016; 149(4): 936-942
- Peled N et al., Non-invasive breath analysis of pulmonary nodules. J Thorac Oncol 2012; 7(10): 1528-1533
- Li XJ et al., A blood-based proteomic classifier for the molecular characterization of pulmonary nodules. Sci Transl Med 2013; 5(207): 207ra142
- Patz EF Jr et al., Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014; 174(2): 269-272
- Horeweg N et al., Lung cancer probability in subjects with CT-detected pulmonary nodules. Thorac Oncol 2013; 8 (Suppl 2; PL03.01)
- Tanner NT et al., The association between smoking abstinence and mortality in the National Lung Screening Trial. Am J Respir Crit Care Med 2016; 193(5): 534-541
- Pastorino U et al., Stopping smoking reduces mortality in low-dose computed tomography (LDCT) screening volunteers. J Thorac Oncol 2015; 10 (suppl 2): PLEN04.07


![Figure 2: National Lung Screening Trial: diminished lung-cancer–related mortality due to low-dose CT screening, as compared to chest X-ray screening [3]](https://memoinoncology.com/wp-content/uploads/2020/04/Grafik-5-preceptorship-vienna-en.jpg)
![Figure 3: Association between loss of FEV1 and probability of lung cancer in men [17]](https://memoinoncology.com/wp-content/uploads/2020/04/Grafik-6-preceptorship-vienna-en.jpg)


