What is new in surgery? Redefining current options
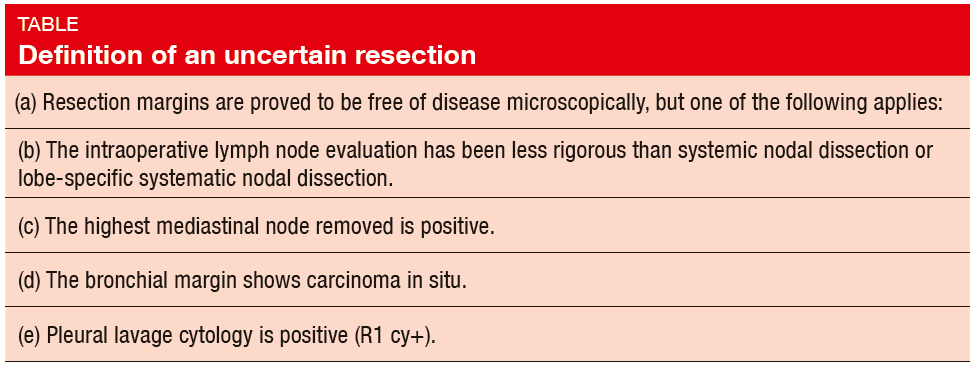
In 2005, the International Association for the Study of Lung Cancer (IASLC) Staging Committee proposed the definition of complete resection of lung cancer, which included the criteria of uncertain resection [1]. Uncertain resection was defined by the criteria detailed in the Table. On behalf of the IA-SLC Staging and Prognostic Factors Committee, Edwards et al. conducted a retrospective analysis of the resection margin status using the data of 14,712 patients obtained from the 8th Edition Database who underwent NSCLC surgery [2]. Full resection status and survival data were available for these patients. Neoadjuvant therapy cases were excluded. Cases were reassigned to R (uncertain) [R(un)], if any of the following applied:
+ Less than 3 N1 or N2 node examined
+ Less than lobe-specific systematic lymph node dissection
+ Extra-capsular invasion of N2 nodes
+ Positive highest lymph node station (status of highest node unavailable)
+ Carcinoma in situ at bronchial resec-tion margin (currently R1 [i. s.])
+ Positive pleural lavage cytology (cur-rently R1 [cy+])
Importance of high-quality surgical staging
Survival curves according to the conventional resection status showed a significant difference between R0 and R1, but no significant difference between R1 and R2. Upon reassignment, 55.8 % of cases (n = 8,203) became R(un) cases. Among the reasons for assignment to the R(un) category, less rigorous intraoperative staging compared to systematic lymph node dissection prevailed in the vast majority of cases, although there was a reasonable number of cases with ,highest station positive only’. In pN2 cases with positivity of the highest station, median survival was 14 months shorter than in the patients who were highest-station–negative (41.0 vs. 55.0 months; HR, 1.45; p < 0.0001). The survival curves according to resection status in N0 cases did separate, but not significantly. In node-positive cases, median survival was 20 months less for patients with R(un) compared to R0 (50.0 vs. 70.0 months; HR, 1.27; p < 0,0001). However, the numbers in the other proposed R(un) categories were small.
The authors concluded that the IA-SLC Proposed Definition for Complete Resection has relevance. It is important to acknowledge that high-quality surgical staging gives the most accurate assignment of stage group and the most favourable survival data, stage by stage, in association with stage migration. However, it is essential that clinical trials take into account the quality standard of surgery, which can be assessed systematically using these criteria. Optimal staging data also allows the most appropriate decision making for routine adjuvant therapy and accurate interpretation of survival in adjuvant therapy clinical trials. The R Domain Sub-Committee will continue work to refine the proposed R status descriptors.
How to handle screen-detected lung cancer
Dr. Shun-ichi Watanabe, Department of Thoracic Surgery, National Cancer Center Hospital, Tokyo, Japan, discussed the optimal management of small tumours detected using CT screening [3]. He reported that the first series of successful segmentectomy was reported in 1973 [4], but the results of the only randomised controlled trial comparing lobectomy with sublobar resection significantly favoured lobectomy [5], thus rendering it the standard surgical approach for more than half a century.
Today, however, many small subsolid tumours are being detected using CT screening. “The ideal procedure, i.e., observation, segmentectomy, or lobectomy, is controversial for some nodules at the moment,” Dr. Watanabe emphasised. In Japan, the type of surgery is selected based on tumour size and C/T ratio, i.e., the maximum consolidation diameter divided by the maximum tumour diameter. “Based on the JCOG0201 trial, a C/T ratio < 0.25 was considered non-invasive,” Dr. Watanabe said [6].
When performing sublobar resection, a choice must be made between segmentectomy and wedge resection. If segmentectomy is performed, the regional lymphatic pathways are removed, which means that anatomic segmentectomy could be applied even for invasive tumours. However, non-anatomic wedge resection should be restricted to non-invasive tumours, as tumour cells might persist within the lymphatic pathways.
Trial results to come
Dr. Watanabe pointed out that in the context of sublobar resection, points of attention relate to ensuring adequate resection margins and the exclusion of tumours with pleural invasion. “Surgical margins should exceed the tumour diameter, which can of course be difficult, particularly in the apical parts of the lung.” In the setting of pleural invasion, skip metastasis is possible.
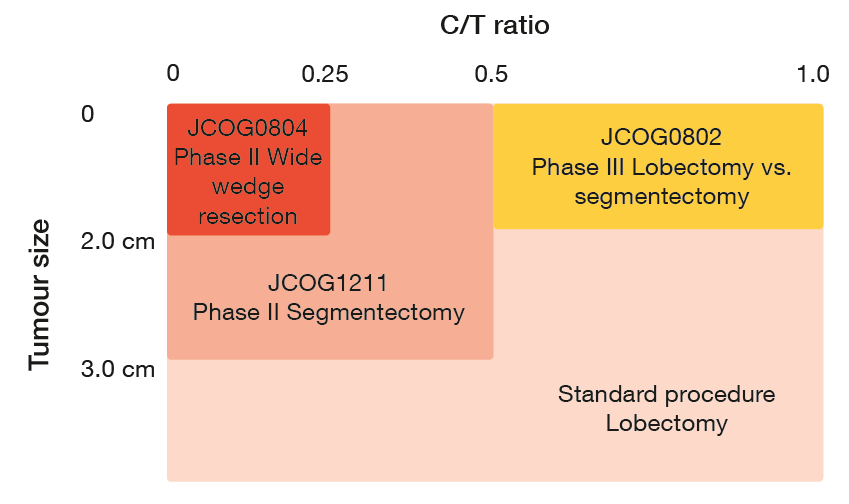
In 2009, the Japan Clinical Oncology Group (JCOG) initiated two clinical trials exploring different surgical approaches for small-sized (≤ 2 cm) lung tumours (Figure 1). The one-arm, phase II JCOG0804 study investigated wide wedge resection, and the phase III JCOG0802 trial compared lobectomy with segmentectomy. “Enrolment has already been completed in both studies, and patients are being followed up.”
JCOG is conducting another phase III trial, JCOG1211, for segmentectomy in patients with T1c tumours sized >2 cm (C/T ratio < 0.5). This study was initiated based on the survival outcomes of JCOG0201 that showed very good prognosis in tumours with a diameter of ≤ 2 cm (C/T ratio < 0.25) and ≤ 3 cm (C/T ratio < 0.5) [7]. Five-year overall survival (OS) rates were 97.1 %and 96.7 %, respectively. JCOG1211 has already completed enrolment. Overall, the three trials on sublobar resection comprise 1,836 cases. These results will be likely to change the textbooks, as Dr. Watanabe concluded.
Figure 1: Ongoing trials exploring sublobar resection
Bilateral mediastinal lymphadenectomy
Experimental studies have shown that lymphatic drainage occurs from the left lower lobe to the contralateral mediastinal nodes [8]. To date, level I evidence on the survival effect of wide mediasti-nal resection is not available, and the role of bilateral mediastinal lymph node dissection in lung cancer remains unknown. Therefore, the aim of a randomised, controlled study was to analyse the impact of bilateral mediastinal lymphadenectomy (BML) on survival in NSCLC patients [9]. Between 2010 and 2013, 89 patients with NSCLC stage I to IIIa were randomised to standard pulmonary resection with either systematic lymph node dissection (SLND; n = 49) or BML (n = 40).
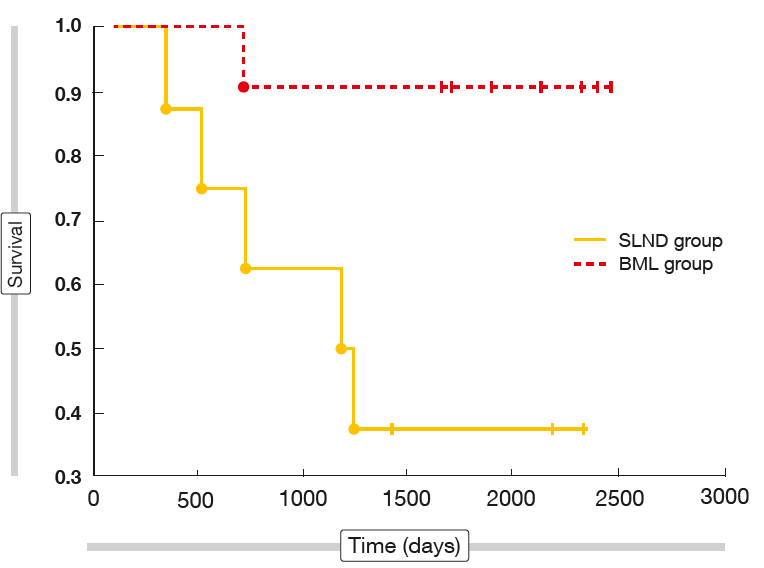
After a mean follow-up of 66.5 months, the 4-year survival rate was significantly higher in the BML group than in the SLND group (72.5 % vs. 51 %; p = 0.039). Separate comparisons were performed for different lobar locations of the tumour, showing no significant differences for 4-year survival rates and mean survival time between the two groups for tumours located in the right lung and those located in the left upper lobe. However, analysis of the left lower lobe revealed significantly improved 4-year survival in the BML cohort (90.9 % vs. 25 %; p = 0.003; Figure 2). Accordingly, mean survival was significantly longer (1,923 vs. 1,244 days; p = 0.027).
These findings indicated that for NSCLC located in the left lower lobe, removal of the contralateral mediastinal lymph nodes might be associated with a significant survival benefit. As patient numbers were low, the trial results should be confirmed in larger randomised controlled studies. A large international trial based on a similar protocol with the aim of validating these findings has recently been launched.
Figure 2: Long-term survival in patients who received standard pulmonary resection with either bilateral mediastinal lymphadenectomy (BML) or systematic lymph node dissection (SLND)
Primary tumour resection in metastatic NSCLC
Oligometastatic NSCLC may represent an indolent phenotype that might benefit from locally ablative treatments such as surgery or radiotherapy. Kang et al. evaluated the potential effect of primary tumour resection and an aggressive local consolidative therapy on 3-year OS and PFS in patients with metastatic OS and progression-free survival (PFS) [10]. Moreover, the objectives included the assessment of surgical outcomes in the treatment of patients with metastatic NSCLC and the identification of clinical factors that predict OS and PFS, in order to improve patient selection for surgery.
Consecutively treated patients with stage IV disease and ≤ 3 metastatic sites were analysed in a retrospective manner. They had received standard first-line systemic therapy (i.e., ≥ 4 cycles of platinum-based doublet chemotherapy) or approved first-line EGFR TKI therapy for ≥ 3 months if the tumour was known to harbour EGFR mutation. According to the extent of pulmonary resection, the patients were divided into two subgroups: intent to cure (ITC; removal of total or primary pulmonary lesions) and intent to biopsy (ITB; preser-vation of major lesions, only diagnostic biopsy via minimally invasive approach). The primary endpoint was 3-year OS and PFS.
Between 2000 and 2015, 115 patients were enrolled. The analysis showed that primary tumour resection in combination with systemic therapy was feasible, tolerable, and significantly extended OS and PFS compared to maintenance therapy or observation alone. Median OS was not reached vs. 23 months with ITC and ITB, respectively (HR, 0.38; p < 0.0001), and median PFS was 36 vs. 10 months (HR, 0.35; p < 0.0001). The ITC cohort experienced both longer OS and PFS across the M1a, M1b and M1c subgroups. Among characteristics evaluated for association with OS and PFS in the multivariate Cox proportional regression analysis, only the clinical M stage and the treatment type (ITC vs. ITB) were identified as significant factors. No patient in either group had grade 4 adverse events (AEs) or died due to an AE. The authors pointed out that these results are exploratory, but worthy of further evaluation. Identification of subgroups of patients who are most likely to benefit is necessary.
Advantages of less invasive surgery
In elderly patients with stage I NSCLC, sublobar resection was shown to be an alternative to standard lobectomy [11]. Laohathai et al. assumed that this approach might be preferable because of reduced operative risk and better preservation of pulmonary function. From 2003 to 2016, 77 octagenarians who underwent curative resection for stage I NSCLC were enrolled. Fifty-three and 24 received lobar and sublobar resection, respectively. The two groups did not differ with regard to sex, smoking history, performance status and comorbidities except for COPD, which was more prevalent in the group treated with sublobar resection. Clinical data were collected retrospectively. OS and recurrence-free survival (RFS) constituted the outcomes, as well as complication rates.
Indeed, OS did not differ significantly between the two groups, with 5-year rates of 51 % and 68 % for lobar and sublobar resection, respectively (p = 0.354). This also applied to RFS (recurrence rates, 57.14 % and 42.86 %, re-spectively; p = 0.623). At the same time, complications occurred less frequently with sublobar resection than with lobar resection (13 % vs. 26 %). Pneumonia and persistent air leak were the predominant AEs in the lobar resection group. The length of hospital stay (LOS) was significantly shorter in patients who received sublobar resection (p = 0.011).
VATS versus OT
Likewise, a retrospective analysis demonstrated equivalence of video-assisted thoracic surgery (VATS) and open thoracotomy (OT) with regard to survival outcomes while revealing a LOS advantage of the less invasive approach [12]. VATS has become the recommended approach for treatment of early-stage lung cancer, but no large randomised clinical trial has formally compared it to OT thus far, although the VIOLET study in the UK is nearing accrual.
This single-institution chart review included a total of 235 patients diagnosed with stage I-III lung cancer who received either VATS or OT between 2005 and 2015. In this group, VATS and OT was performed in 101 and 134 cases, respectively. Age at diagnosis, sex, tobacco use, tumour location, and tumour size were comparable across the groups. No significant difference occurred with respect to the risk of positive resection margins for VATS vs. OT. OS and RFS were similar for both techniques (p = 0.68 and p = 0.23, respectively), while median LOS was significantly shorter in patients receiving VATS (4 vs. 6 days; p = 0.002). These favourable outcomes were achieved regardless of tumour stage at diagnosis.
REFERENCE
- Rami-Porta R et al., Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005; 49(1): 25-33
- Edwards J et al., The IASLC Lung Cancer Staging Project: analysis of resection margin status and proposals for R status descriptors for non-small cell lung cancer. WCLC 2017, PL 02.06
- Watanabe S et al., What is the optimal management of screen-detected lung cancers? WCLC 2017, PL 01.04
- Jensik RJ et al., Segmental resection for lung cancer. A fifteen-year experience. J Thorac Cardiovasc Surg 1973; 66: 563-572
- Ginsberg RJ, Rubinstein LV, Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995; 60: 615-623
- Suzuki K et al., A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011; 6: 751-756
- Asamura H et al., Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg 2013; 146: 24-30
- Hata E et al., Rationale for extended lymphadenectomy for lung cancer. Theor Surg 1990; 5: 19-25
- Kuzdzal J et al., Randomised trial of systematic lymph node dissection versus bilateral mediastinal lymphadenectomy in patients with NSCLC – the Cracow Study. WCLC 2017, OA 04.01
- Kang X et al., Primary tumor resection versus maintenance therapy of observation for patients with metastatic non-small cell lung cancer in combination with first-line systemic therapy. WCLC 2017, OA 04.03
- Laohathai S et al., Comparison between sublobar and lobar resection in octagenarians with pathologic stage I non-small cell lung cancer. WCLC 2017, P1.16-005
- Shaheen S et al., Less is more – video assisted thoracic surgery (VATS) vs. open thoracotomy in the management of resectable lung cancer. WCLC 2017, P1.16-006