Emerging standards in tumours with rare genetic drivers
First-line brigatinib: ALTA-1L
In the setting of ALK-positive NSCLC, the first-generation ALK inhibitor crizotinib is currently being replaced as the first-line standard by next-generation agents. The open-label, randomised, multicentre, phase III ALTA-1L trial investigated the ALK/ROS1 inhibitor brigatinib in untreated patients. Brigatinib, which has excellent CNS activity, was administered at a daily dose of 180 mg after a 7-day lead-in at 90 mg in the experimental arm (n = 137), whereas patients enrolled in the control arm received crizotinib 250 mg twice daily (n = 138). ALK positivity was defined using multiple ALK diagnostic tests, which reflects the real-world setting. Approximately 30 % of patients in each arm had asymptomatic brain metastases at baseline. One line of prior chemotherapy was allowed. Overall, 27 % of patients had received chemotherapy in the locally advanced or metastatic setting.
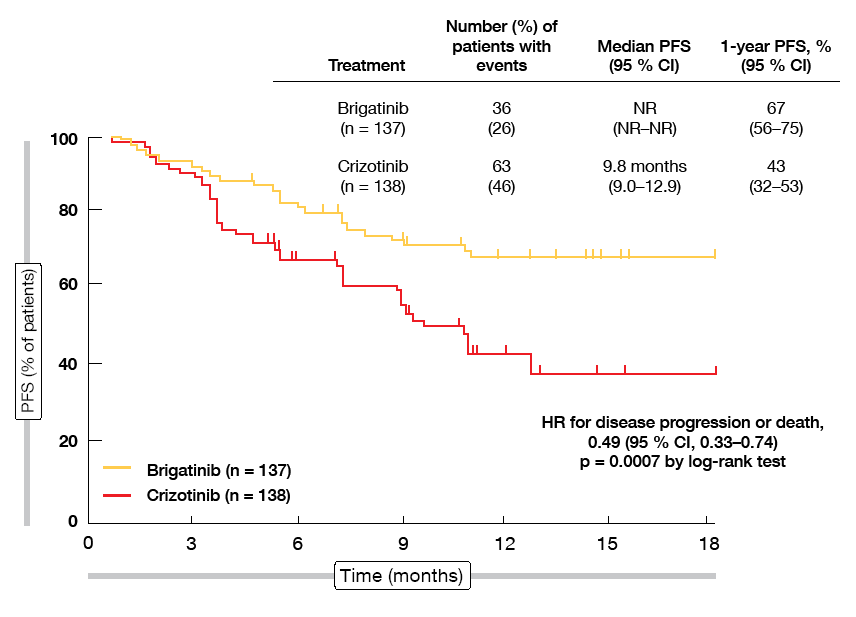
At the WCLC 2018, Camidge et al. presented the first pre-planned interim analysis of the ALTA-1L trial [1]. After a follow-up of 9 to 11 months, the study had already met its primary endpoint. Brigatinib was superior to crizotinib with respect to PFS according to a blinded independent review committee (not reached vs. 9.8 months; HR, 0.49; p = 0.0007; Figure 1). At 12 months, 67 % vs. 43 % of patients were progression-free. Brigatinib treatment gave rise to more favourable PFS in both patients with prior chemotherapy (not reached vs. 11.0 months; HR, 0.35; p = 0.0207) and those without (not reached vs. 9.8 months; HR, 0.55; p = 0.0095).
Figure 1: Progression-free survival by blinded independent review committee with brigatinib vs. crizotinib in ALTA-L1
CNS activity of brigatinib
The subgroup analysis suggested that reductions in the risk of progression or death were greater for patients with baseline CNS disease than for those without (HRs, 0.20 and 0.72, respectively). However, as the PFS dataset was more mature in patients with brain lesions, particularly for the crizotinib arm that had a greater number of CNS events, this finding preferentially emphasised CNS progression among patients with baseline brain disease as an earlier differentiating event. Additional follow-up will reveal the full differential impact of the two drugs on both early and later-onset progression events.
ORRs did not differ significantly between the two treatment arms (71 % vs. 60 %; p = 0.0678). The median duration of response had not been reached for brigatinib (vs. 11.1 months), with a 12-month probability of maintaining response of 75 % vs. 41 %. Among patients with measurable CNS lesions, brigatinib demonstrated a significantly higher intracranial response rate of 78 % (vs. 29 %; OR, 10.42; p = 0.0028). When including those with non-measurable CNS disease, the odds ratio improved to 13.00 (67 % vs. 17 %; p < 0.0001). Also, intracranial PFS differed to a highly statistically significant degree in favour of brigatinib (not reached vs. 5.6 months; HR, 0.27; p < 0.0001).
Brigatinib was well tolerated, with dose reductions being mainly protocol-mandated for asymptomatic laboratory abnormalities such as elevations of creatine phosphokinase, lipase, and amylase. Excess AEs observed with crizotinib treatment, on the other hand, included gastrointestinal effects, transaminase elevations, bradycardia, oedema, and visual effects. Although interstitial lung disease or pneumonitis occurred in both arms, early-onset pneumonitis that emerged within 14 days of treatment initiation appears to be a unique side effect of brigatinib, but only occurred in 3 %, which is half the rate seen in the post-crizotinib setting [2]. The authors concluded that brigatinib represents a promising new first-line treatment option for ALK-positive NSCLC.
MET exon 14-positive NSCLC: tepotinib
Approximately 3 % of NSCLC cases harbour MET proto-oncogene that causes exon 14 to be skipped during processing of mRNA [3, 4]. Tepotinib has been developed as a highly selective oral inhibitor of MET. Interim data from the single-arm, phase II VISION trial investigating tepotinib in patients with advanced NSCLC and MET exon skipping 14 alterations suggest encouraging activity of tepotinib 500 mg daily [5]. Patients were treated in the first, second and third lines. The efficacy and safety analyses included 40 and 46 patients, respectively. MET exon skipping 14 mutation status was positive in liquid biopsy in 60.9 %, in tumour biopsy in 80.4 %, and in both in 43.5 %.
Objective responses occurred in 35.0 % and 57.5 % according to independent review committee and investigator, respectively. Disease control was achieved in 62.5 % and 72.5 %, respectively. Responses lasted for a median of 14.3 months, although these data are not mature yet. Teponitib was well tolerated, with a median time on treatment of 4.7 months. The most common AEs included peripheral oedema and diarrhoea, which were of mild or moderate intensity in the majority of cases. In 15.2 %, patients discontinued treatment due to AEs. Recruitment to the trial is ongoing.
Crizotinib in MET exon 14-alterations
Apart from its effects on ALK and ROS1, crizotinib is also a potent MET inhibitor. The multicentre phase I PROFILE 1001 trial examined crizotinib 250 mg twice daily in an expansion cohort of patients with MET exon 14-altered advanced NSCLC without prior exposure to MET-directed targeted therapy. According to an updated analysis conducted in 65 patients, crizotinib treatment proved active with an ORR of 32 % [6]. Three patients (5 %) developed complete responses. Median duration of response was 9.1 months, and median PFS amounted to 7.3 months. OS data were not mature at the time of data cut-off.
A vital part of the analysis was an exploratory analysis of local molecular profiling results, as MET exon 14-positive cancers are molecularly diverse, with a wide array of different mutation types occurring at different sites. Up to 20 % harbour concurrent MET amplification. This analysis demonstrated therapeutic benefits despite heterogeneity with respect to both mutation type and absence or presence of concurrent MET amplification, which was found in 7 %. The overall safety profile of crizotinib in this subset was consistent with that previously described for ALK– and ROS1-rearranged lung cancer. The investigators noted that screening for MET exon 14 alterations in the clinic is important. As shown in this trial, alterations can be detected successfully using comprehensive tumour or plasma profiling. Crizotinib recently received Breakthrough Designation by the US Food and Drug Administration (FDA) for the treatment of MET exon 14-altered lung cancers.
Entrectinib as a new option for ROS1-positive cancer
ROS1 fusions are driver mutations in 1 % to 2 % of NSCLC cases [7, 8]. CNS disease represents an unmet need in ROS1-positive patients; crizotinib is the current standard of care, but progression commonly develops in the CNS as the first site in treated patients. The oral ROS1/NTRK/ALK TKI entrectinib was designed to cross the blood-brain barrier and remain within the CNS. Moreover, preclinical studies showed that entrectinib inhibits ROS1 more potently than crizotinib [9].
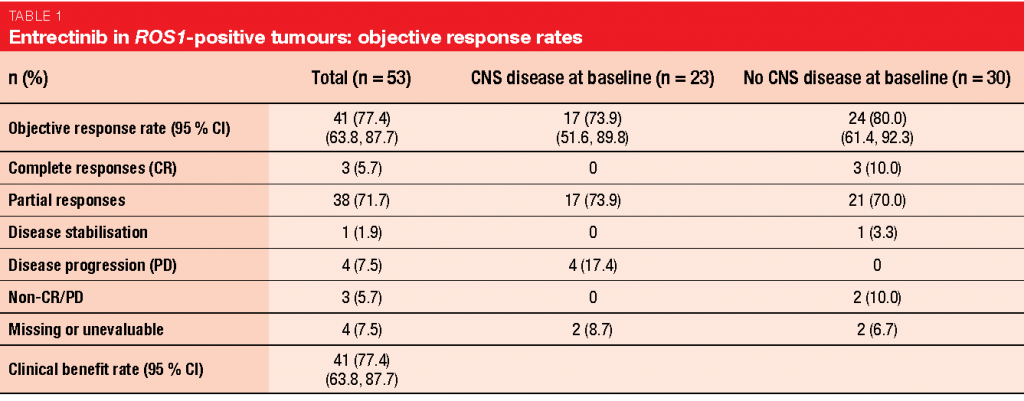
An integrated analysis of 3 studies (STARTRK-2, STARTRK-1, ALKA-372-001) conducted with entrectinib in a total of 53 patients with ROS1-positive NSCLC illustrates the efficacy of this treatment [10]. In patients with and without CNS metastases at baseline, clinically meaningful, deep and durable systemic responses were obtained. The ORR amounted to 77.4 %; for patients with and without CNS disease, this was 73.9 % and 80.0 %, respectively (Table 1). Median duration of response was 24.6 months. Intracranial responses occurred in 55 % of patients with brain metastases and lasted for a median of 12.9 months; here, 20 % experienced complete responses. In the total cohort, PFS was 19.0 months. Patients with and without CNS lesions had a PFS of 13.6 and 26.3 months, respectively. Entrectinib was tolerable, with a manageable safety profile. Most of the AEs were managed with dose interruption or dose reduction. Only 3.9 % of treatment-related AEs led to discontinuation.
BRAF-positive tumours: vemurafenib monotherapy
Approximately 2 % of NSCLC cases carry BRAF mutations as their driver aberration [11]. BRAF inhibitors are recommended for these patients in most guidelines. In addition to combination therapy consisting of dabrafenib and trametinib, single-agent treatment with the BRAF inhibitors dabrafenib or vemurafenib is an option for patients who do not tolerate combination therapy. In this context, the French National Cancer Institute launched a programme permitting nationwide access to vemurafenib for patients with BRAF-mutated tumours. At the WCLC 2018, Mazières et al. reported the findings obtained in the NSCLC cohort that included patients with metastatic NSCLC progressing after ≥ 1 standard treatment [12]. They had BRAF V600 or other BRAF mutations as assessed by direct sequencing or next-generation sequencing in authorised molecular genetic centres and had not received any prior BRAF- or MEK-targeted treatment. Hundred patients with V600 mutations were analysed; the group with non-V600 mutations comprised 15 individuals. Overall, this cohort resembled a real-world population due to pronounced pre-treatment and reduced performance status in a considerable percentage of patients. Brain metastases were allowed if treated.
ORR, the primary endpoint, was analysed using a sequential Bayesian approach. In the BRAF V600 cohort, the analysis showed that vemurafenib 960 mg twice daily provided reasonable responses with a mean Bayesian estimated success rate of 44.9 %. Responses lasted for 6.4 months. PFS and OS were 5.2 and 9.3 months, respectively. Patients with non-V600 mutations, on the other hand, did not benefit from the vemurafenib treatment (mean Bayesian estimated success rate, 5.9 %; median PFS, 1.8 months; median OS, 5.2 months). The safety profile proved manageable, with asthenia, decreased appetite, acneiform dermatitis and nausea constituting the most common AEs. Twenty-seven patients stopped the treatment due to toxicity.
Based on these findings, the authors concluded that single-agent vemurafenib can be considered if the combination of dabrafenib and trametinib, which remains the preferred option due to comparatively higher response rates, is not well tolerated or cannot be used in countries where the combination has not yet been approved. These results emphasise the need of integrating BRAF V600 in routine biomarker screening
Robust activity of RET inhibitor in heavily pre-treated patients
In solid tumours, RET is an established oncogene that is activated by either fusions or mutations. In NSCLC, RET fusions are present in approximately 2 % of patients. The potent and selective RET inhibitor LOXO-292 showed robust anti-tumour activity in RET-fusion-positive, locally advanced or metastatic NSCLC in the phase I LIBRETTO-001 trial that enrolled 38 NSCLC patients across 8 dose levels [13]. Most of them had received prior chemotherapy or immunotherapy, or both. The median number of prior systemic regimens was 3.
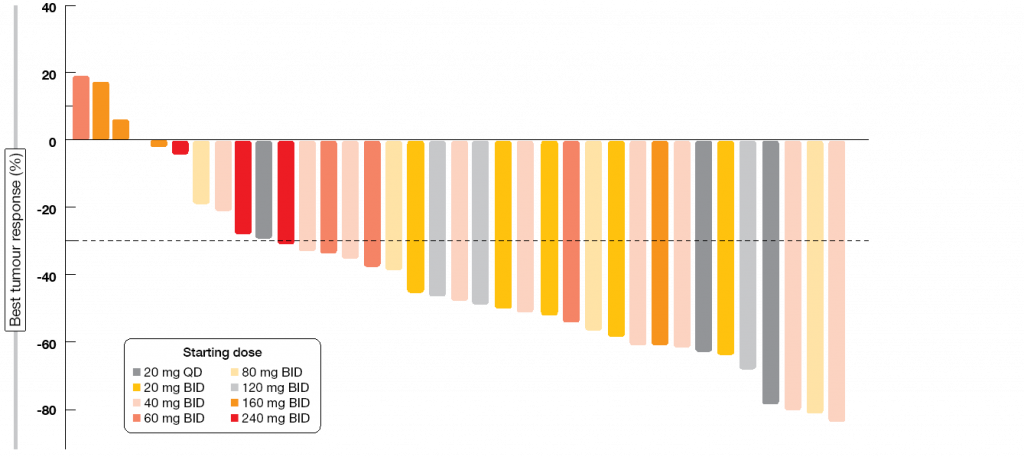
Sixty-eight percent of patients responded to LOXO-292. RECIST 1.1 responses occurred at all starting dose levels prior to any intra-patient dose escalation (Figure 2). Treatment activity was independent of prior therapy. Four patients with measurable CNS disease participated in the trial; all of them experienced intracranial responses. At the time of the analysis, almost all of the responding patients remained on therapy, with 92 % of responses ongoing. The majority of these had been ongoing for ≥ 6 months. Consistent with the highly selective drug design, the treatment showed high safety and tolerability. LOXO-292 was granted Breakthrough Therapy Designation by the FDA in September 2018. Phase II assessments are currently ongoing in multiple cohorts.
Figure 2: Responses to treatment with LOXO-292 in patients with RET-fusion–positive NSCLC
REFERENCES
- Camidge DR et al., Brigatinib vs crizotinib in patients with ALK inhibitor-naive advanced ALK+ NSCLC: first report of a phase 3 trial (ALTA-1L). WCLC 2018, PL02.03
- Kim DW et al., Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol 2017; 35(22): 2490-8
- Schrock AB et al., Characterization of 298 patients with lung cancer harboring MET exon 14 skipping alterations. J Thorac Oncol 2016; 11(9): 1493-502
- Paik PK et al., Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discovery 2015; 5(8): 842-9
- Felip E et al., Phase II data for the MET inhibitor tepotinib in patients with advanced NSCLC and METexon 14-skipping mutation. WCLC 2018, OA12.01
- Drilon A et al., Updated antitumor activity and safety of crizotinib in patients with MET exon 14-altered advanced non-small cell lung cancer. WCLC 2018, OA12.02
- Bergethon K et al., ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012; 30(8): 863-70
- Dugay F et al., Clinicopathological characteristics of ROS1- and RET-rearranged NSCLC in caucasian patients: Data from a cohort of 713 non-squamous NSCLC lacking KRAS/EGFR/HER2/BRAF/PIK3CA/ALK alterations. Oncotarget 2017; 8(32): 53336-51
- Rolfo C et al., Entrectinib: a potent new TRK, ROS1, and ALK inhibitor. Expert Opin Investig Drugs 2015; 24(11): 1493-500
- Doebele RC et al., Efficacy and safety of entrectinib in locally advances or metastastic ROS1-positive non-small cell lung cancer (NSCLC). WCLC 2018, OA02.01
- Barlesi F et al., Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016; 387(10026): 1415-26
- Mazières J et al., Vemurafenib in patients harboring V600 and non V600 BRAF mutations: final results of the NSCLC cohort from the AcSé trial. WCLC 2018, OA12.05
- Oxnard GR et al., Clinical activity of LOXO-292, a highly selective RET inhibitor, in patients with RET fusion+ non-small cell lung cancer. An update from ASCO 2018. WCLC 2018, OA12.07