What is new in SCLC?
Lurbinectedin plus irinotecan
A novel approach for targeting lung tumors with small-cell histology consists in the inhibition of transactivated transcription, as small-cell lung cancer (SCLC) has been found to be a transcription-addicted malignancy [1]. Rudin et al. defined four molecular SCLC subtypes according to their differential expression of four key transcription regulators [2]. Lurbinectedin, which acts by selectively inhibiting oncogenic transcription and modulating the tumor microenvironment, has been granted accelerated approval by the US FDA in June 2020 for the treatment of patients with metastatic SCLC who experienced disease progression on or after platinum-based chemotherapy based on the results of a phase II study [3].
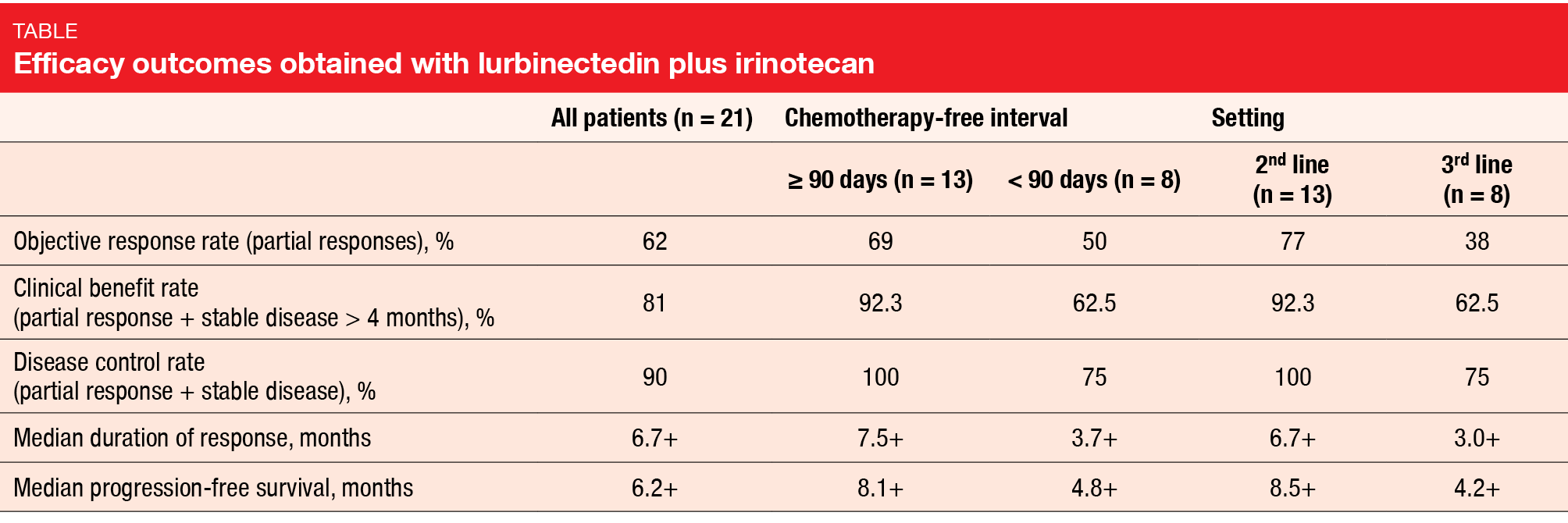
As preclinical observations suggested synergism of lurbinectedin and irinotecan, a phase IB/II trial investigating escalating dose regimens was initiated in patients with various cancer types. At WCLC 2020, Ponce et al. presented the findings for 21 SCLC patients included in Cohort A who received lurbinectedin 2 mg/m2 on day 1 plus irinotecan 75 mg/m2 on days 1 and 8 [4]. In addition, G-CSF support was administered. Eighty-one percent of these 21 patients had extensive-stage SCLC. Bulky disease was present in 29 %, and 24 % showed CNS metastases. Thirty-eight percent had received two prior lines of treatment for advanced disease. While 71 % of patients had developed complete or partial responses to prior platinum therapy, 19 % had been refractory to this treatment.
Particular benefits in poor-prognosis settings
Lurbinectedin plus irinotecan demonstrated remarkable anti-tumor activity. Sixty-two percent of patients experienced partial remissions, and disease control resulted in 90 % (Table). Median PFS and median duration of response were 6.2 and 6.7 months, respectively. Notable efficacy was observed in patients with poor prognosis, such as those with resistant disease, as indicated by a short chemotherapy-free interval, and those treated in the third-line setting (Table). Also, patients with brain metastases responded to the combination.
Toxicity was shown to be transient and manageable. AEs included mostly grade 1 and 2 events, although grade 3/4 neutropenia occurred in 61.9 %, and grade 3/4 febrile neutropenia was reported in 9.5 % despite G-CSF prophylaxis. Apart from hematological abnormalities, diarrhea frequently emerged (all grades, 33.3 %; grade 3/4, 28.6 %), as did fatigue (all grades, 66.7 %; grade 3/4, 23.8 %). No patient discontinued treatment due to toxicity, and there were no drug-related deaths. Dose reductions were required in 52.4 %. Considering these findings, the authors noted that further development of lurbinectedin plus irinotecan is warranted in patients with SCLC. The SCLC cohort of this phase I/II study is currently being expanded to 47 patients.
IMpower133: patients reaching maintenance
In the phase I/III IMpower133 study, atezolizumab plus carboplatin/etoposide (CP/ET) followed by maintenance therapy with atezolizumab has given rise to significant improvements in OS and PFS compared to placebo plus CP/ET followed by placebo maintenance [5]. The exploratory analysis reported at WCLC 2020 assessed the benefit of atezolizumab vs. placebo in the group that reached the maintenance phase of IMpower133 [6].
This population included patients who received at least the first dose of maintenance therapy regardless of the number of chemotherapy cycles administered. Similar proportions of patients met this criterion in the two arms (experimental arm: n = 154, 77 %; control arm: n = 164, 81 %). The baseline characteristics were balanced between these groups. A generalized linear model was used to identify characteristics that might be prognostic or predictive of reaching the maintenance phase. Also, the researchers used a multivariate Cox model from the start of maintenance treatment to evaluate the treatment effect on OS and PFS to account for potential lead-time bias.
Three prognostic factors for reaching the maintenance phase were identified. These included younger age (odds ratio, 0.459), favorable ECOG performance status (OR, 0.439), and lower LDH levels (OR, 0.589). Moreover, a significant treatment interaction was demonstrated for age (p = 0.004). The mortality risk was reduced by 41 % in the experimental arm compared to the chemotherapy-only arm (HR, 0.59). Median OS from the start of maintenance amounted to 12.5 vs. 8.4 months; when assessed from the start of randomization, this was 15.7 vs. 11.3 months. Likewise, median PFS was longer from the start of maintenance (2.6 vs. 1.8 months) and from randomization (5.5 vs. 4.5 months), with a 36 % risk reduction (HR, 0.64). Patients across the treatment arms showed similar safety results despite the continuation of atezolizumab monotherapy in the maintenance phase. In their conclusion, the authors stated that both the induction treatment with atezolizumab plus CP/ET and the atezolizumab maintenance appeared to contribute to the OS benefit observed in IMpower133.
REFERENCES
- Christensen CL et al., Targeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitor. Cancer Cell 2014; 26(6): 909-922
- Rudin CM et al., Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer 2019; 19: 289-297
- Trigo J et al., Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol 2020; 21(5): 645-654
- Ponce S et al., Efficacy and safety profile of lurbinectedin-irinotecan in patients with relapsed SCLC. WCLC 2020, OA11.04
- Horn L et al., First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018; 379(23): 2220-2229
- Reck M et al., IMpower133: exploratory analysis of maintenance therapy in patients with extensive-stage small cell lung cancer. WCLC 2020, OA11.06
© 2020 Springer-Verlag GmbH, Impressum
More posts
Interview: Lung cancer screening: hurdles in daily routine and in the research laboratory
Interview: Lung cancer screening: hurdles in daily routine and in the research laboratory