Immune-based strategies are raising hope in small-cell tumors
DeLLphi-300: tarlatamab
Especially after frontline chemoimmunotherapy, treatment options are limited in patients with small-cell lung cancer (SCLC). Notch ligand delta-like ligand 3 (DLL3) represents a potential therapeutic target as it is aberrantly expressed on the surface of SCLC cells [1, 2]. By binding both DLL3 and CD3, the bispecific T cell engager (BiTE©) tarlatamab induces T-cell–mediated tumor lysis [3]. Tarlatamab is the first DLL3-targeted immunotherapy to undergo clinical evaluation.
In the first-in-human DeLLphi-300 study, tarlatamab was tested in the setting of relapsed/refractory SCLC with the aim to assess safety and tolerability, determine the maximum tolerated dose or recommended phase II dose, characterize pharmacokinetics, and to investigate preliminary antitumor activity. The patients had progressed or recurred following ≥ 1 platinum-based chemotherapy (including a PD-L1 inhibitor, if standard of care) and had ≥ 2 measurable lesions. Tarlatamab doses of 0.003-100 mg and 100 mg Q2W were used in the dose exploration and expansion phases, respectively.
At WCLC 2022, Borghaei et al. presented exploration and first expansion data of the DeLLphi-300 study for a total of 106 patients [4]. One third of these had received ≥ 3 prior lines of therapy, and half had previously been treated with anti-PD-(L)1 agents. In almost all cases, extensive-stage disease was present at the initial diagnosis.
Low-grade AEs and promising efficacy
Tarlatamab showed a manageable safety profile across the evaluated doses. Cytokine release syndrome (CRS) was the most common treatment-related AE (all grades, 53 %) but was almost exclusively restricted to mild and moderate events (grade ≥ 3, 1 %). CRS occurred mainly in cycle 1 and was generally manageable. No grade 4/5 CRS events were reported. Eight percent of patients required tocilizumab treatment for the management of this complication.
Similarly, higher-grade events were rare among the other common AEs including pyrexia (all grades, 38 %; grade ≥ 3, 2 %), dysgeusia (23 % and 0 %, respectively), fatigue (22 % and 3 %, respectively), and nausea (20 % and 0 %, respectively). Treatment-related neurologic events, which occurred in 50 %, were predominantly grade 1 and mainly comprised dysgeusia and headache. Confusion constituted the most common grade ≥ 3 and the only grade 4 neurologic event in the study. All-grade and grade 4 treatment-related neutropenia emerged in 16 % and 4 %, respectively. No patient developed febrile neutropenia. Only 4 % of the study population discontinued tarlatamab therapy due to treatment-related AEs.
In this heavily pretreated patient group, tarlatamab demonstrated promising antitumor activity with encouraging response durability. The confirmed overall response rate (ORR) was 23 % and included 2 cases of complete response. Thirty-seven percent of patients experienced target lesion shrinkage ≥ 30 %. Responses lasted for a median of 13.0 months. Median progression-free survival (PFS) and median overall survival (OS) were 3.7 and 13.2 months, respectively. Based on these results, the registrational phase II DeLLphi-301 study is assessing tarlatamab in SCLC patients after ≥ 2 lines of treatment (NCT05060016).
Long-term survivors in KEYNOTE-604
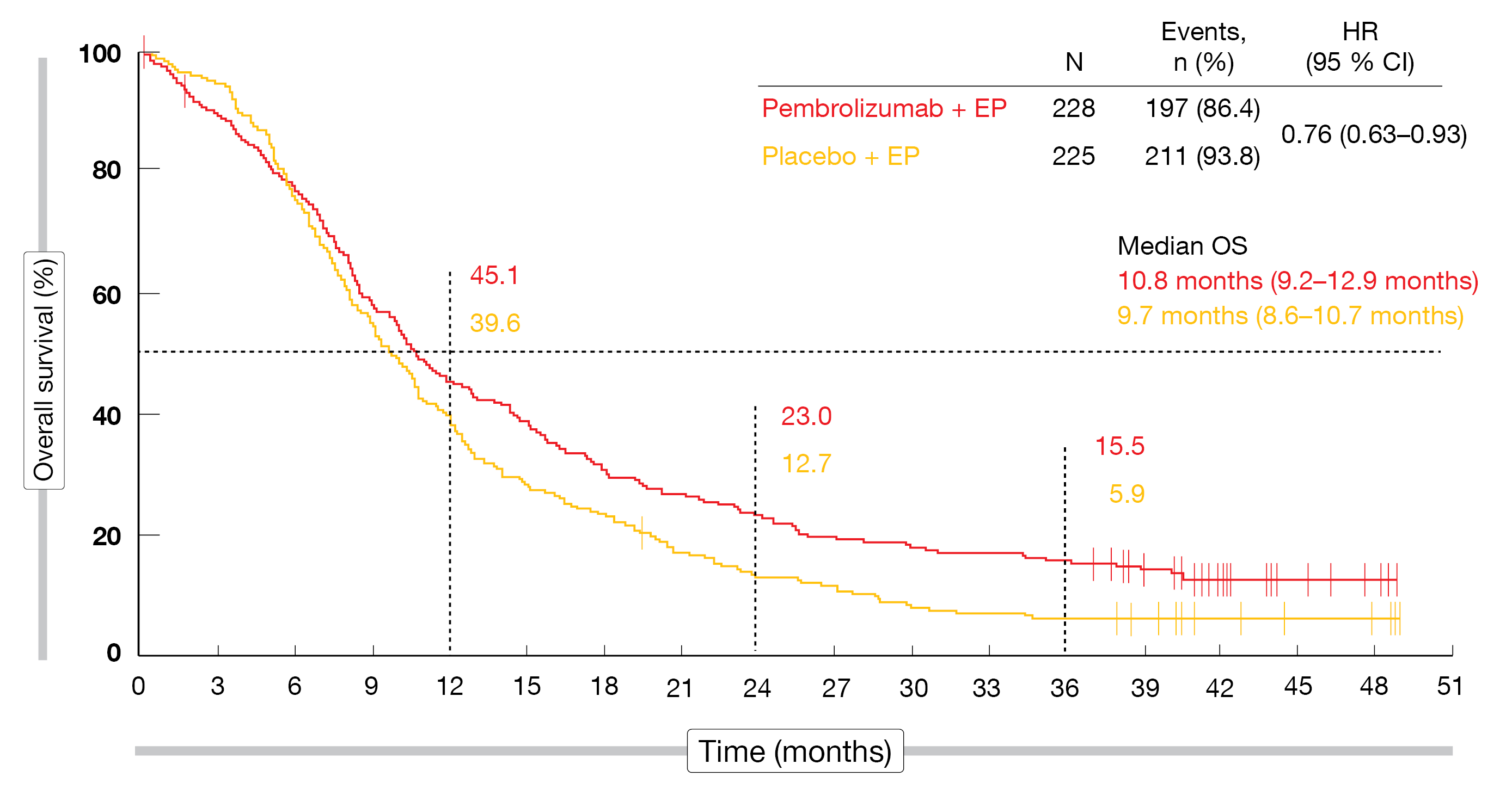
In the setting of previously untreated stage IV SCLC, pembrolizumab plus etoposide/platinum (EP) Q3W for 4 cycles followed by pembrolizumab for up to 31 cycles significantly improved PFS compared to placebo plus EP followed by placebo in the phase III KEYNOTE-604 study [5]. The experimental and control arms included 228 and 225 patients, respectively. Rudin et al. presented long-term results after approximately 3.5 years of follow-up, as well as outcomes in patients who completed 35 cycles of pembrolizumab [6].
Pembrolizumab plus chemotherapy continued to elicit clinically meaningful OS and PFS improvement. The OS analysis in the ITT population revealed a 24 % mortality reduction with the pembrolizumab-based treatment (median, 10.8 vs. 9.7 months; HR, 0.76) even though 15 % of patients in the control arm had subsequently received immune checkpoint inhibitor therapy (Figure). At 36 months, the OS rates were approximately 3 times higher in the experimental arm (15.5 % vs. 5.9 %). The risk of progression or death was reduced by 30 %, with median PFS of 4.8 vs. 4.3 months (HR, 0.70) and 36-month PFS rates of 6.9 % vs. 0.5 %. All subgroups except for patients with baseline brain metastases benefited from the immunotherapy-based approach with respect to both OS and PFS. Ten percent of the total group responded for ≥ 42 months. As previously reported, the safety profile of pembrolizumab plus EP was generally manageable. Immune-mediated AEs occurred in 27.4 % vs. 12.1 %, with grade 3-5 rates of 8.1 % vs. 1.3 %.
Eighteen patients had completed 35 cycles of pembrolizumab. At the time of the last assessment, 14 remained alive. Median OS had not been reached yet; the 2-year OS rate after completing the 35 cycles was 72.2 %. All patients were responders, with 11.1 % and 88.9 % achieving complete and partial remissions, respectively. In 83.3 %, responses lasted for ≥ 24 months. Among the control patients, on the other hand, only 2 of 225 individuals (0.9 %) had completed 35 cycles and were alive at data cutoff.
These results support the continued assessment of pembrolizumab-based combinations for the treatment of extensive-stage SCLC. The phase III KEYVIBE-008 trial is investigating MK-7684A, a co-formulation of vibostolimab, and pembrolizumab plus EP vs. atezolizumab plus EP in the first-line setting (NCT05224141).
Figure: Updated overall survival with pembrolizumab plus chemotherapy vs. placebo plus chemotherapy in the KEYNOTE-604 study
REFERENCES
- Leonetti A et al., Notch pathway in small-cell lung cancer: from preclinical evidence to therapeutic challenges. Cell Oncol (Dordr) 2019; 42(3): 261-273
- Saunders LR et al., A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 2015; 7(302): 302ra136
- Giffin MJ et al., AMG 757, a half-life extended, DLL3-targeted bispecific T-cell engager, shows high potency and sensitivity in preclinical models of small-cell lung cancer. Clin Cancer Res 2021; 27(5): 1526-1537
- Borghaei H et al., Phase 1 updated exploration and first expansion data for DLL3-targeted T-cell engager tarlatamab in SCLC (DeLLphi-300 study). WCLC 2022, OA12.05
- Rudin CM et al., Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol 2020; 38(21): 2369-2379
- Rudin CM et al., First-line pembrolizumab or placebo combined with etoposide and platinum for ES-SCLC: KEYNOTE-604 long-term follow-up results. WCLC 2022, OA12.06
© 2022 Springer-Verlag GmbH, Impressum