Stage I-III disease: surgical and systemic options
SLR as new standard of care in cT1a N0 (≤ 2 cm)
Lobar resection has been the surgical standard of care for cT1 N0 non–small-cell lung cancer (NSCLC) for decades, while sublobar resection (SLR) was reserved for a subset of patients with marginal pulmonary reserve. However, the recently published JCOG0802/WJOG4607L study showed that in fit patients with cT1a N0 tumors sized ≤ 2 cm, segmentectomy was not inferior to lobectomy regarding overall survival (OS) [1]. The randomized, international, non-inferiority, phase III CALGB 140503 (Alliance) trial compared lobectomy with SLR (i.e., segment or wedge resection) in patients with peripheral NSCLC cT1a N0 ≤ 2 cm. Preoperatively, node negativity at level 10 and ≥ 2 mediastinal nodal stations had to be confirmed. Approximately 350 patients were randomly allocated to each arm. In the SLR group, 58.8 % underwent wedge resection.
Indeed, the two approaches were demonstrated to be equal [2]. Regarding the primary endpoint of disease-free survival (DFS), the 5-year rates were 64.1 % vs. 63.6 % for the lobar resection and SLR groups, respectively, after a median follow-up of 7 years (HR, 1.01; p = 0.0176 for non-inferiority). SLR was not inferior to lobectomy across major demographic and clinical features according to a post-hoc exploratory subgroup analysis. Moreover, no differences emerged in terms of lung cancer-related recurrences/deaths (HR, 0.99; p = 0.9521) or competing deaths (HR, 1.12; p = 0.5897). The 5-year OS rates amounted to 78.9 % vs. 80.3 % (p = 0.014 for non-inferiority).
Disease recurrence was observed in approximately 30 % of patients without significant differences between arms in the incidence of isolated locoregional or systemic relapses. Regarding pulmonary function, SLR, as compared to lobectomy, induced less pronounced reductions in FEV1 and FVC, although the authors noted that this might not be clinically meaningful. Overall, the results of the Alliance study and the JCOG0802/WJOG4607L trial establish SLR as the standard of care for patients with NSCLC cT1a N0 sized ≤ 2 cm.
NADIM II: secondary endpoints
In the randomized phase II NADIM II study, neoadjuvant nivolumab plus chemotherapy has been shown to significantly improve pathological complete response (pCR) rates compared to chemotherapy alone in patients with resectable stage IIIA-B lung cancer (36.8 % vs. 6.9 %; OR, 7.88; p = 0.0068) [3]. Patients who had R0 surgery went on to receive adjuvant treatment with nivolumab Q4W for 6 months in the experimental arm (n = 57) or observation in the control arm (n = 29). At WCLC 2022, Provencio et al. presented the results for the secondary endpoints of the trial [4].
In the total group, lobectomy constituted the most frequently performed surgical procedure (78.1 %). Definitive surgery was achieved in 93.0 % vs. 69.0 % with the immunotherapy-based treatment vs. chemotherapy alone (OR, 5.96; p = 0.00807). Also, the results for R0 resection favored the combined regimen (92.5 % vs. 65.0 %; OR, 6.60; p = 0.007), as did the downstaging rates (69.8 % vs. 40.0 %; OR, 3.47; p = 0.04). Median progression-free survival (PFS) had not been reached yet in the experimental arm after a median follow-up of 26.1 months, while it was 18.3 months in the control arm (HR, 0.48; p = 0.025). At 24 months, 66.6 % vs. 42.3 % of patients were progression-free. The PFS analysis by pCR status showed that patients with pCR in both arms remained progression-free over time, whereas those with pathological incomplete responses had drastically lower PFS probability.
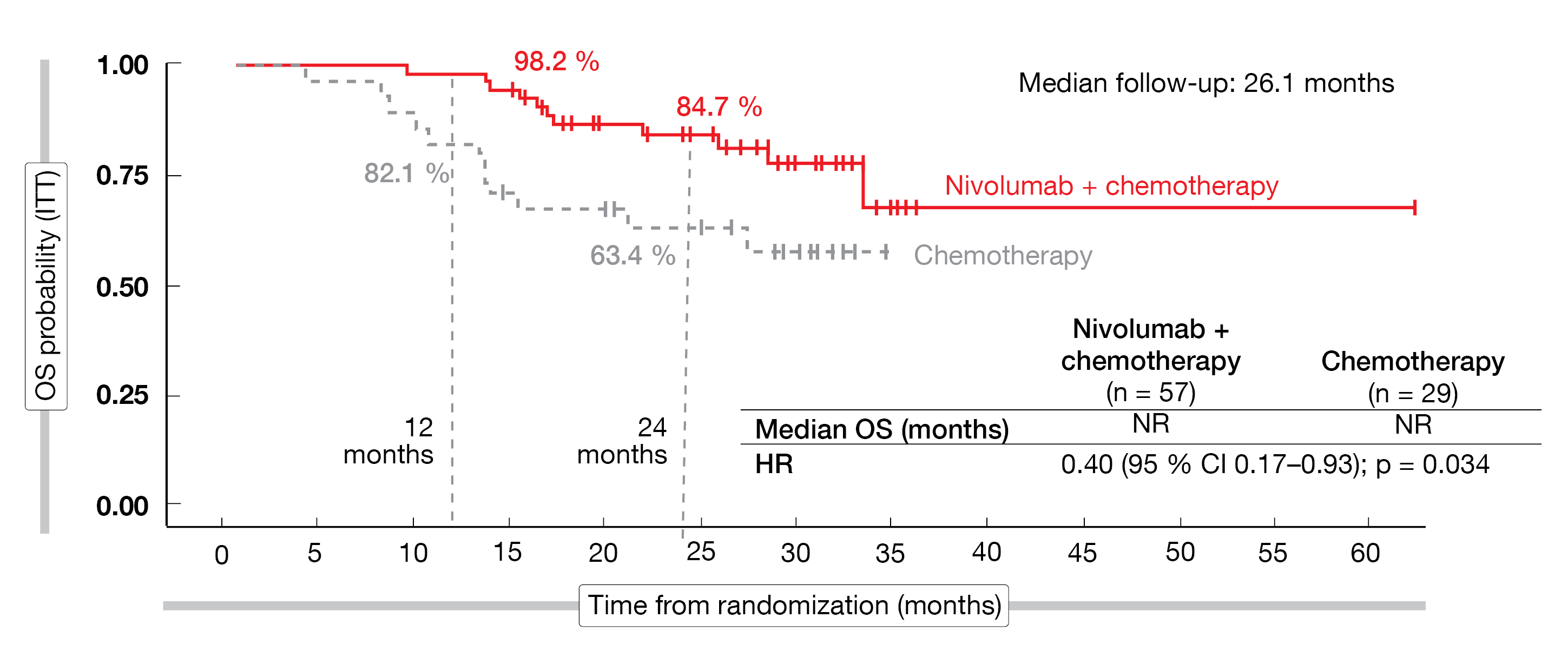
Median OS had not been reached in either arm yet, although the 24-month rates significantly favored the nivolumab-based strategy (84.7 % vs. 63.4 %; HR, 0.40; p = 0.034; Figure 1). Again, patients who obtained pCR did not experience any event irrespective of the type of treatment; the groups with incomplete responses, on the other hand, fared considerably worse. Overall, the addition of nivolumab did not impede the feasibility of surgery, and the combination maintained a tolerable safety profile. As the authors emphasized, NADIM II is the first clinical trial with a neoadjuvant immunotherapy-based combination for resectable stage IIIA-B NSCLC to show improved OS.
According to a biomarker analysis of the NADIM II data, pre-treatment circulating tumor DNA (ctDNA) levels significantly predicted OS and PFS [5]. This was true regardless of the cut-off used. The authors concluded that baseline ctDNA levels clearly identified patients at high risk of progression and death, thus adding a significant degree of prognostic information to the clinical stage.
Figure 1: NADIM II: overall survival with neoadjuvant nivolumab plus chemotherapy vs. chemotherapy alone
First OS analysis of IMpower010
Adjuvant atezolizumab has been demonstrated to significantly prolong DFS compared to best supportive care in the phase III IMpower010 trial that included patients with completely resected stage IB-IIIA (IB tumors ≥ 4 cm) NSCLC who had received 1–4 cycles of platinum-based chemotherapy [6]. Felip et al. reported the first pre-specified interim analysis of OS and a safety analysis at a median follow-up of 45.3 months [7]. According to the hierarchical testing protocol of IMpower010, the stage II-IIIA subpopulation with PD-L1 ≥ 1 % was analyzed first.
In this group, a trend for OS was seen favoring atezolizumab. Median OS had not been reached yet in either arm, with 60-month OS rates of 76.8 % vs. 67.5 % (HR, 0.71). Regarding other subpopulations, the PD-L1 ≥ 50 % stage II-IIIA cohort experienced a clinically meaningful OS trend (HR, 0.43), while the all-randomized stage II-IIIA cohort and the ITT population (stage IB-IIIA) did not derive any survival benefit from the checkpoint inhibitor treatment. Patients with PD-L1 ≥ 50 % stage II-IIIA disease in whom EGFR/ALK aberrations had been excluded exhibited 60-month OS rates of 84.8 % vs. 67.5 % (HR, 0.42).
After 13 months of additional follow-up, the safety profile remained broadly unchanged, and no new safety signals emerged. All-grade and grade 3/4 AEs of special interest for atezolizumab occurred in 52.1 % and 7.9 %, respectively. These numbers matched the rates reported at the time of the DFS interim analysis [6]. In their entirety, the updated findings support the favorable benefit-risk profile of adjuvant atezolizumab in PD-L1–positive, resected NSCLC and contribute to evidence supporting the use of this standard-of-care regimen. IMpower010 will continue on to the final DFS analysis.
DOLPHIN: durvalumab plus radiotherapy
Based on the PACIFIC study, consolidation therapy with durvalumab after definitive chemoradiotherapy has been established as the standard of care for locally advanced, unresectable NSCLC [8]. However, approximately 25 % of these patients are unable to receive durvalumab due to AEs of chemoradiotherapy or poor performance status [9]. The multicenter, single-arm, phase II DOLPHIN study evaluated a chemotherapy-free approach consisting of durvalumab 10 mg/kg Q2W plus concurrent curative radiation therapy (60 Gy) for up to 1 year until disease progression in patients with PD-L1–positive, unresectable stage III or postoperatively recurrent NSCLC. Tachihara et al. presented the findings for 35 patients with ECOG performance status of 0 or 1 after a median follow-up of 18.7 months [10].
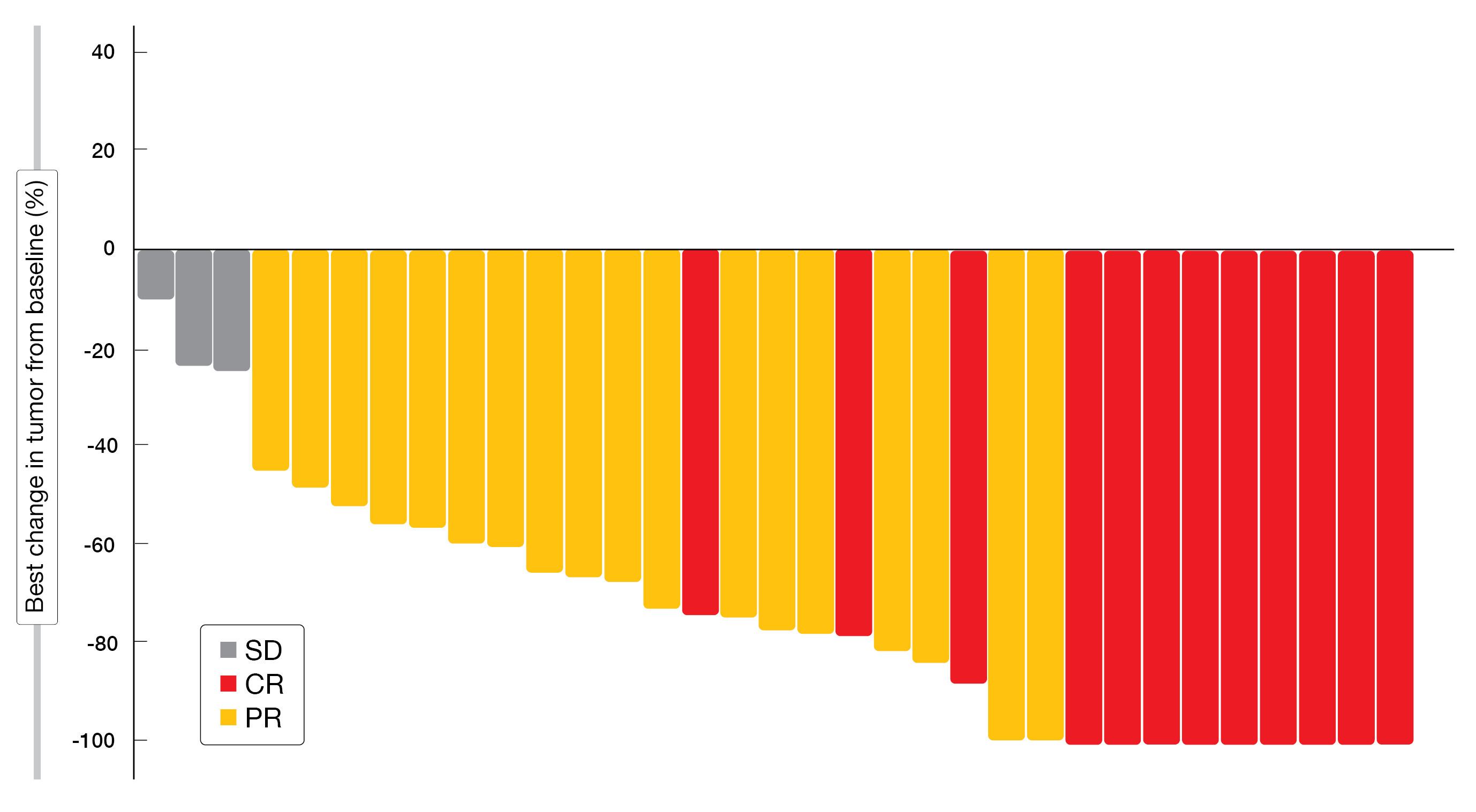
The PFS rate at 12 months by independent central review, which was defined as the primary endpoint, was 72.1 %, thus exceeding the expected 50 % rate. Overall, 90.9 % of patients responded to treatment, with 36.4 % and 54.5 % achieving complete and partial remissions, respectively (Figure 2). All of the participants obtained disease control. Grade 3/4 AEs occurred in 47.1 %, and 2 patients (5.9 %) died due to AEs. In 17.6 %, AEs led to the discontinuation of durvalumab, although all patients completed radiotherapy. Any-grade pneumonitis or radiation pneumonitis were reported in 61.8 %, with grade 3/4 events in 11.8 %. No patient died because of pneumonitis or radiation pneumonitis. The authors concluded that durvalumab plus radiotherapy is promising and warrants phase III assessment.
Figure 2: Responses achieved with durvalumab and concurrent radiotherapy in the DOLPHIN study
Surgery vs. chemoradiation in T4 N2 disease
No clear treatment guidelines exist for T4 N2 NSCLC with additional intrapulmonary nodules. Using data entered into the National Cancer Database between 2010 and 2015, Kumar et al. evaluated long-term OS in the setting of T4 N2 M0 lung cancer with additional nodules in a different ipsilateral lobe [11]. The retrospective analysis compared 196 patients after multimodal therapy including primary site resection (thoracic surgery group) with 277 patients who had received concurrent chemoradiation without surgery (chemoradiation group). Patients in the thoracic surgery group were somewhat younger than those in the chemoradiation group (65.5 vs. 67 years), and higher percentages were female (60.2 % vs. 45.8 %) and had adenocarcinoma histology (68.1 % vs. 47.6 %).
The analysis yielded improved survival in the thoracic surgery group, with 5-year rates of 40.3 % vs. 26.8 % (p < 0.001). The propensity score matched analysis showed 5-year survival rates of 45.8 % vs. 20.4 % (p < 0.001); after adjustment for factors including age, sex, comorbidity score, median census-tract household education level and tumor location, the mortality risk was reduced by 45 % (HR, 0.55; p < 0.001). Adjusted hazard ratios for thoracic surgery vs. chemoradiation were 0.54 for patients with tumors sized ≤ 3 cm (p = 0.030) and 0.47 for patients aged ≤ 65 years without comorbidities (p = 0.048). Within the thoracic surgery cohort, 51 and 145 patients had received induction chemotherapy prior to surgery and adjuvant chemotherapy after surgery, respectively, each with or without radiation. No significant difference resulted in 5-year OS between these two groups (41.6 % vs. 36.4 %; p = 0.23). This also applied after adjustment for patient and disease characteristics (p = 0.37).
In their summary, the authors point out that these findings further highlight the heterogeneity of stage IIIB disease and support consideration of NSCLC with additional nodules as a unique subcategory of T4 N2 disease. Further prospective studies could evaluate surgery as part of a multimodal treatment regimen in patients with T4 N2 NSCLC who have additional intrapulmonary nodules in a different ipsilateral lobe.
REFERENCES
- Saji H et al., Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022; 399(10335): 1607-1617
- Altorki N et al., Lobar or sub-lobar resection for peripheral clinical stage IA ≤ 2 cm NSCLC: results from an international randomized phase III trial (CALGB 140503 [Alliance]). WCLC, PL03.06
- Provencio-Pulla M et al., Nivolumab + chemotherapy versus chemotherapy as neoadjuvant treatment for resectable stage IIIA NSCLC: primary endpoint results of pathological complete response (pCR) from phase II NADIM II trial. J Clin Oncol 40, 2022 (suppl 16; abstr 8501)
- Provencio M et al., Nivolumab + chemotherapy vs. chemotherapy as neoadjuvant treatment for resectable IIIA-B NSCLC. WCLC 2022, PL03.12
- Romero A et al., Pre-treatment ctDNA levels significantly predict OS and PFS in the NADIM II trial. WCLC 2022, MA06.03
- Felip E et al., Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021; 938(10308): 1344-1357
- Felip E et al., IMpower010: overall survival interim analysis of a phase III study of atezolizumab vs best supportive care in resected NSCLC. WCLC 2022, PL03.09
- Antonia SJ et al., Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017; 377(20): 1919-1929
- Saito G et al., Real-world survey of pneumonitis and its impact on durvalumab consolidation therapy in patients with non-small cell lung cancer who received chemoradiotherapy after durvalumab approval (HOPE-005/CRIMSON). Lung Cancer 2021; 161: 86-93
- Tachihara M et al., Phase II study of durvalumab plus concurrent radiotherapy in unresectable locally advanced NSCLC – DOLPHIN Study (WJOG11619L). WCLC 2022, MA06.04
- Kumar A et al., Multimodal management of T4, N2 non-small-cell lung cancer with additional ipsilateral pulmonary nodules. WCLC 2022, OA02.04
© 2022 Springer-Verlag GmbH, Impressum