New data on EGFR-directed TKIs across 3 generations
Erlotinib plus bevacizumab
EGFR TKI treatment has become a standard first-line strategy for patients with advanced, EGFR-mutation–positive NSCLC. Established agents include the first-generation drugs gefitinib and erlotinib, the second-generation agents afatinib and dacomitinib, and the third-generation TKI osimertinib. Combinations of EGFR TKIs with other drug classes might lead to outcome optimisation, for instance the additional administration of anti-angiogenic drugs, such as bevacizumab and ramucirumab.
The NEJ026 trial was the first phase III study to investigate first-line erlotinib in combination with the anti-VEGF antibody bevacizumab [1]. In the phase II setting, the randomised JO25567 trial already showed a significant PFS benefit of erlotinib plus bevacizumab compared to erlotinib monotherapy [2]. The NEJ026 study was conducted to confirm these results. Japanese patients with non-squamous, stage IIIB/IV or postoperatively recurrent NSCLC with activating EGFR mutations received either bevacizumab 15 mg/kg Q3W plus erlotinib 150 mg once daily (QD; n = 112) or erlotinib alone (n = 112). After disease progression, patients in the experimental arm were treated with platinum and pemetrexed, followed by pemetrexed maintenance, while those in the control arm received platinum plus pemetrexed and bevacizumab, followed by maintenance with pemetrexed and bevacizumab. Asymptomatic central nervous system (CNS) metastases were allowed and present in 32.1 % in each arm.
PFS by independent review committee constituted the primary endpoint. According to the pre-planned interim analysis for PFS, the addition of bevacizumab led to a significant PFS prolongation (16.9 vs. 13.3 months; HR, 0.605; p = 0.01573) [1]. Patients with both exon 19 deletion and exon 21 L858R mutation benefited from the combination. ORR by independent review did not differ significantly across the treatment arms (72.3 %. vs. 66.1 %). Hypertension, proteinuria and haemorrhages occurred more frequently in the bevacizumab arm, but proved manageable. As the investigators pointed out, erlotinib plus bevacizumab represents a new standard first-line treatment in the setting of EGFR-mutant NSCLC. Biomarker analyses and OS follow-up are ongoing.
Concurrent use of gefitinib and chemotherapy
In the NEJ002 trial, gefitinib treatment gave rise to a PFS benefit compared to standard chemotherapy (10.8 vs. 5.4 months; HR, 0.30; p < 0.001) [3], although no significant difference resulted for OS. Also, only 70 % of patients in the gefitinib arm received platinum-doublet chemotherapy, which is a standard post-TKI treatment. Therefore, a thorough use of both EGFR TKI treatment and chemotherapy was expected to improve OS.
Indeed, the phase II NEJ005 study suggested promising efficacy of the concurrent use of gefitinib and chemotherapy compared to a sequential regimen [4]. In the phase III setting, the NEJ009 trial evaluated the concurrent administration of gefitinib QD plus carboplatin and pemetrexed for 4 to 6 cycles [5]. Maintenance treatment after the induction phase contained daily gefitinib plus pemetrexed Q3W until progression. Patients in the control arm, on the other hand, received gefitinib QD until progression; at that time, a platinum-based second-line regimen was recommended.
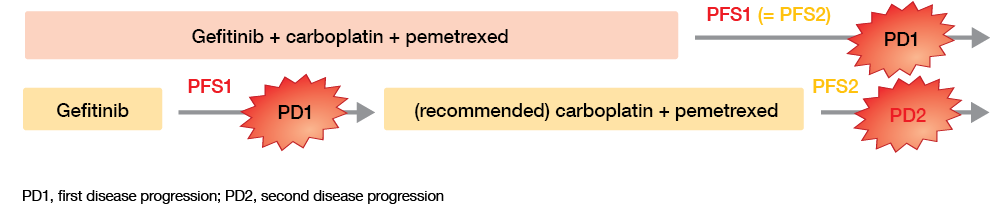
Multiple primary endpoints were evaluated; these included PFS, PFS2 (i.e., a PFS comparison at the time of the second disease progression [PD2] in the reference arm and the first progression [PD1] in the experimental arm; Figure), and OS. Across Japan, 345 patients with non-squamous, previously untreated stage IIIB/IV or recurrent NSCLC were enrolled at 47 institutions.
Figure: PFS outcomes as defined in the NEJ009 study
Time to first progression counts
As expected, the combination treatment was superior by a wide margin with regard to PFS1 (20.9 vs. 11.2 months; HR, 0.494; p < 0.001) and ORR (84.0 % vs. 67.4 %). These effects are most likely due to the considerably longer gefitinib treatment exposure in the experimental arm (730 vs. 462 days). At the time of PD1, the clinical status of patients (i.e., ECOG PS, number of metastatic organs, brain metastasis) was comparable across the trials arms. This was not the case at PD2, however; here, patients treated sequentially fared much worse. The PFS2 analysis yielded no difference between the two regimens (20.9 vs. 20.7 months; HR, 0.966; p = 0.774).
An additional OS analysis revealed a significant advantage of the combination regimen (52.2 vs. 38.8 months; HR, 0.695; p = 0.013). No difference resulted for survival from PD1 (19.3 vs. 23.0 months; HR, 1.037; p = 0.812) even though the majority of patients in the gefitinib-alone arm had received a platinum regimen after the first progression. This indicates that OS in this study is closely related to PFS1, rather than to PFS2. The authors concluded that prolongation of the time until the first progression is critical for patients with EGFR-mutant NSCLC, and that PFS is a good surrogate marker for OS.
Haematological AEs were more common in the combination arm. However, only few patients discontinued treatment due to toxicities in both treatment groups. Overall, the addition of carboplatin and pemetrexed to gefitinib significantly improved PFS and OS in untreated advanced EGFR-mutant NSCLC, with acceptable toxicity. The combined regimen might therefore be an effective first-line option in this setting.
OS results obtained in ARCHER 1050
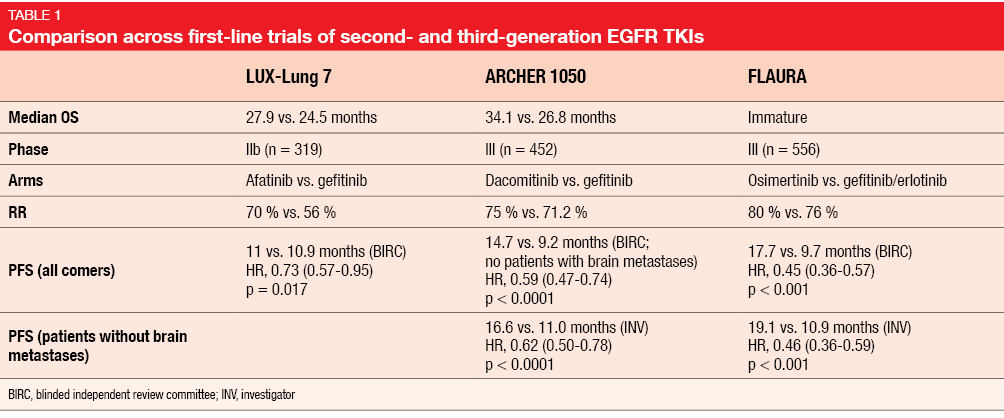
The second-generation, irreversible EGFR TKI dacomitinib was investigated in the phase III, randomised, open-label ARCHER 1050 study. Wu et al. reported significant PFS improvement with dacomitinib versus gefitinib as first-line treatment in patients with EGFR-mutant, advanced NSCLC (14.7 vs. 9.2 months; HR, 0.59; p < 0.0001) [6]. A total of 452 patients received either dacomitinib 45 mg QD or gefitinib 250 mg QD. Pre-existing CNS metastases were not allowed in this trial, as the efficacy of dacomitinib in patients with brain lesions was unknown at the time of study inclusion. Seventy to 80 % of the population in both arms were Asian. Approximately 60 % were younger than 65 years.
The pre-specified final OS analysis of ARCHER 1050 presented at the ASCO 2018 Congress showed that this is the first randomised phase III trial comparing two first-line EGFR TKIs to demonstrate OS improvement [7]. Median OS results were 34.1 vs. 26.8 months for dacomitinib and gefitinib, respectively (HR, 0.76; p = 0.0438). At 30 months, 56.2 % vs. 46.3 % of patients were alive. The subgroup analysis yielded no OS differences between the two treatments in patients with exon 19 deletion (HR, 0.88; p = 0.4862) or exon 21 L858R mutation (HR, 0.707; p = 0.0805), although the study was not powered to capture survival differences in these subgroups. Likewise, the OS analysis for Asian patients did not show a significant treatment benefit (HR, 0.812; p = 0.1879). Median OS in the dacomitinib-treated patients who went on to receive third-generation EGFR TKI treatment (9.7 % of the population) was 36.7 months. Other EGFR TKIs as subsequent therapy led to an OS of 34.7 months.
The increased EGFR-inhibitory activity of dacomitinib caused typical AEs, with grade ≥ 3 acneiform dermatitis occurring in 13.7 %, grade ≥ 3 diarrhoea in 8.8 %, and grade ≥ 3 paronychia in 7.5 %. Gefitinib, on the other hand, only gave rise to slightly higher rates of grade ≥ 3 liver enzyme elevations. AEs frequently made dose modifications necessary in the experimental arm (66.5 % vs. 8.0 % with gefitinib). The authors concluded that dacomitinib should be considered as a new option for the first-line management of patients with EGFR-mutant advanced NSCLC.
Putting the ARCHER 1050 data into perspective
As Dr. Daniel Tan, National Cancer Centre Singapore, pointed out in his discussion of the ARCHER 1050 and NEJ009 data, here are two phase III trials finally demonstrating OS benefit over a standard EGFR TKI, although questions remain [8]. All of the prior EGFR TKIs were approved for first-line treatment based on PFS, but showed no significant differences in OS, which was potentially due to crossover. When comparing ARCHER 1050 with the other key first-line trials investigating second- and third-generation EGFR TKIs, Dr. Tan noted that the LUX-Lung 7 study, which tested afatinib against gefitinib [9], had a smaller sample size (n = 319) than ARCHER 1050 (n = 452; Table 1). Also, this was a phase IIb study, which might have confounded its ability to detect an OS difference. While patients with brain metastases were included in LUX-Lung 7, this was not the case for ARCHER 1050. Long-term tolerability of dacomitinib is a potential concern, raising the need to define the optimal pharmacologically active dose. Another unsolved issue is sequencing of second- and third-generation TKIs.
Also, the question remains of where osimertinib fits. OS data from the FLAURA trial are not mature yet, and resistance mechanisms are still incompletely characterised, with uncertain druggability. Dr. Tan emphasised that EGFR-mutant lung cancer is a clinically and genomically heterogeneous disease. Initial upfront therapy can augment its natural history; therefore, it is increasingly important to ascertain the individual risk of disease progression with a view to rationalising upfront therapy. According to Dr. Tan, there is a need to critically evaluate the risk-benefit ratio of these potential standards of care and to tailor them to individual patient preference.
Real-world evidence on afatinib
Tolerability-guided dose adjustment of afatinib reduced the incidence and severity of AEs without affecting efficacy in the LUX-Lung studies. Halmos et al. reported the impact of afatinib dose modifications on the efficacy and safety of this treatment in a real-world setting [10]. A total of 228 patients from 13 countries who received first-line afatinib were included in this non-interventional, observational study.
As in the LUX Lung trials, afatinib dose adjustments reduced the frequency and intensity of AEs without impacting efficacy. Time on treatment and time to progression were similar regardless of dose modifications or reduced starting dose. Sixty-seven percent of patients who started on doses of ≥ 40 mg underwent reductions, with 86 % of these occurring in the first 6 months. In 12 %, doses were increased. AEs constituted the main reason for modifications. Dose adjustments were more frequent in females, older patients, Eastern Asian patients, and those with lower body weight. The analysis revealed no new safety signals. These results highlighted the benefit of tailoring afatinib dose based on individual patient characteristics and AEs to optimise outcomes.
Clinical outcomes obtained with afatinib in real-world practice were the focus of a Japanese analysis of 128 patients 76 of whom received first-line afatinib, while 52 were treated in the re-challenge setting [11]. The use of afatinib gave rise to comparable or even better outcomes than in previous trials. In the first-line setting, PFS and OS were 17.8 months and 39.5 months, respectively. Although dose reductions became necessary in 58 patients (76.3 %) due to AEs, this did not affect OS outcomes (39.5 months in the reduction group vs. not yet reached in the group without reduction; p = 0.37). Moreover, patients whose doses were reduced even experienced longer PFS than those in whom this was not the case (18.0 vs. 7.9 months; p = 0.016). The ORR was 64 %. In the re-challenge setting, the analysis yielded an ORR of 24 %.
Afatinib in uncommon EGFR mutations
A retrospective, multicentre study evaluated the efficacy of afatinib in patients with lung adenocarcinoma harbouring uncommon EGFR mutations in Spanish clinical practice [12]. Medical records of 67 NSCLC patients who had been treated with afatinib between 2012 and 2017 at 23 Spanish institutions were reviewed. Eighty percent of patients had received afatinib as first-line therapy. Uncommon EGFR mutations were analysed as complex mutations (Group A; n = 20), exon 20 insertion (Group B; n = 23), or single mutations (Group C; n = 24).
No differences in clinical characteristics emerged across the three groups. Eighteen percent of patients started afatinib at reduced doses, and 24 % required dose reductions. Responses to afatinib were significantly higher in Groups A and C (70 % and 54 %, respectively) than in Group B (13 %; pairwise comparison p < 0.001 and p = 0.013, respectively). Median OS for the entire cohort was 19.9 months, with HRs of 0.27 (p = 0.009) and 0.40 (p = 0.037) for Groups A and C compared to Group B, respectively. As the authors stated in their conclusion, afatinib was active in NSCLC with uncommon EGFR mutations in clinical practice, particularly in complex and single mutations. Further strategies, however, are called for in patients with exon 20 insertion.
Neoadjuvant use: the ASCENT trial
Afatinib in the neoadjuvant setting was assessed in the phase II ASCENT study conducted in patients with stage III, EGFR-mutant NSCLC whose disease burden was feasible for chemoradiation [13]. After treatment with afatinib 40 mg QD for 2 months, patients received chemoradiation and went on to undergo resection or adjuvant therapy with afatinib 40 mg QD for 2 years, provided no disease progression had occurred with the neoadjuvant TKI use. After surgery, adjuvant chemotherapy was optional, followed by adjuvant afatinib.
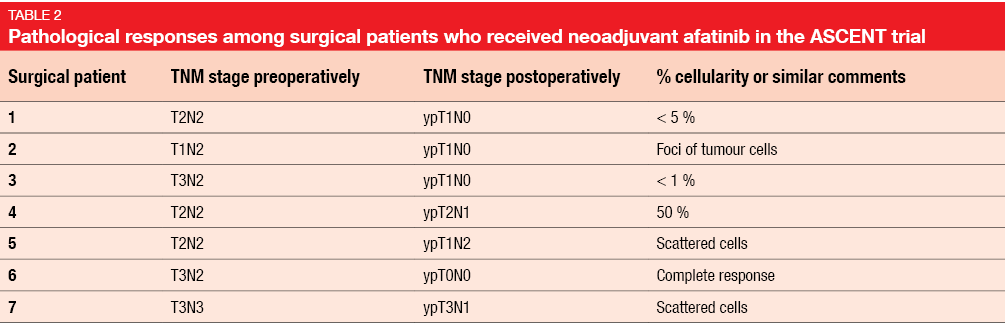
Although only 13 patients were included in this analysis, it showed a favourable neoadjuvant response rate of 75 %. The delivery of chemoradiation with or without surgery was not impeded by the neoadjuvant afatinib administration. Six out of 7 patients who underwent surgery experienced clinically significant pathological responses (i.e., scant cells, < 5 % cellularity; Table 2). Median PFS was 34.8 months, with 7 patients staying recurrence-free at the time of the analysis. This result compares favourably to the 16.8-month PFS outcome observed in the immunotherapy arm of the PACIFIC study [14], supporting the genotype-directed approach. PACIFIC had tested sequential treatment with the PD-L1 inhibitor durvalumab versus placebo in locally advanced, unresectable stage III NSCLC. The investigators noted that the feasibility of 2 months of neoadjuvant afatinib administration might exceed adjuvant TKI use. Accrual to the ASCENT trial is continuing.
Significance of TMB for anti-EGFR treatment
Tumour mutation burden (TMB) might have multiple biological implications that depend on the specific treatment and disease setting. For immunotherapy, the relationship between TMB and improved treatment benefit has been described. Offin et al. hypothesised that TMB might have a distinct (and inverse) relationship with outcomes in the setting of targeted therapies, where TMB might be a surrogate for the presence of resistance pathways [15]. The researchers identified 153 patients with EGFR-mutant (exon 19 deletion and exon 21 L858R mutation) lung cancer treated with first- and second-generation EGFR TKIs, who had next generation sequencing with the tumour-profiling multiplex panel MSK-IMPACTTM performed on pre-treatment tissue. OS and time to treatment discontinuation (TTD) with the initial EGFR TKI therapy were evaluated according to TMB in univariate and multivariate analyses.
The results indicated that EGFR-mutant lung cancers have a broad molecular heterogeneity with a wide range of TMB even within this specific oncogene-defined disease. As the investigators had surmised, TMB was inversely associated with the efficacy of the EGFR TKI treatment. Both OS and TTD were shortest in the group showing high TMB, with HRs of 0.49 and 0.57, respectively, according to multivariate analysis (p = 0.025 and 0.009, respectively). This relationship is in contrast to that seen with immunotherapy, which highlights the varied and context-specific implications of TMB.
Resistance to osimertinib: what’s new?
The third-generation EGFR TKI osimertinib has shown significant clinical activity in the phase III AURA3 study when compared to platinum-pemetrexed chemotherapy for patients with T790M-positive NSCLC after progression on first- or second-generation EGFR TKI treatment [16]. Moreover, in comparison to erlotinib or gefitinib in the first-line setting, osimertinib gave rise to a significant PFS benefit in the phase III FLAURA trial [17].
Despite the increasing role of osimertinib for treatment of NSCLC, there is limited data regarding resistance mechanisms to this agent. However, a comprehensive understanding of these mechanisms is required in order to develop strategies to overcome osimertinib resistance. Le et al. therefore performed an analysis based on the MD Anderson Lung Cancer Moon Shot GEMINI database and the Moffitt electronic health record, Clinical Genomic Action Committee database and pyrosequencing database for NSCLC patients with EGFR T790M mutation, isolating those who were treated with osimertinib [18]. A total of 118 patients met the study criteria. Almost all of them (95 %) had received previous EGFR TKI treatment, mostly erlotinib.
Genomic profiling showed that resistance mechanisms are diverse, with T790M preservation and T790M loss each prevailing in approximately half of cases. In T790M-preserved samples, C797S/L792H mutations were found (58 %), as well as MET amplifications. This means that tertiary mutation of EGFR is a common mechanism in this group. In those with T790M loss, the resistance mechanisms were largely EGFR-independent and non-oncogene-driver–mediated (i.e., PIK3CA mutation, MET amplification, SCLC transformation). Cell cycle gene alterations showed an association with inferior clinical outcomes.
Zhang et al., when analysing differences relating to the mutation spectrum in 110 patients with activating EGFR mutations who were clinically osimertinib-resistant, observed that acquired mutations leading to osimertinib resistance were more likely to be identified in the group with deletion 19 than in patients with L8585R mutation (62.5 % vs. 39.1 %; p = 0.015) [19].
Prediction of response to osimertinib
Previous longitudinal analyses from the AURA programme suggested that early clearance of plasma EGFR mutations in patients with T790M-positive advanced NSCLC receiving osimertinib is a prognostic marker for improved PFS [20]. Shepherd et al. investigated whether the presence of plasma EGFR mutations in patients from AURA3 at 3 and 6 weeks after starting osimertinib therapy is associated with clinical outcomes [21].
It was shown that osimertinib-treated patients with detectable EGFR mutations in their baseline plasma samples, which are indicative of tumour shedding, had less favourable outcomes than those without shedding regarding both PFS (8.3 vs. 14.0 months) and ORR (68 % vs. 75 %). The researchers concluded that detectable tumour shedding might reflect increased disease burden and could be a prognostic biomarker for poorer outcome.
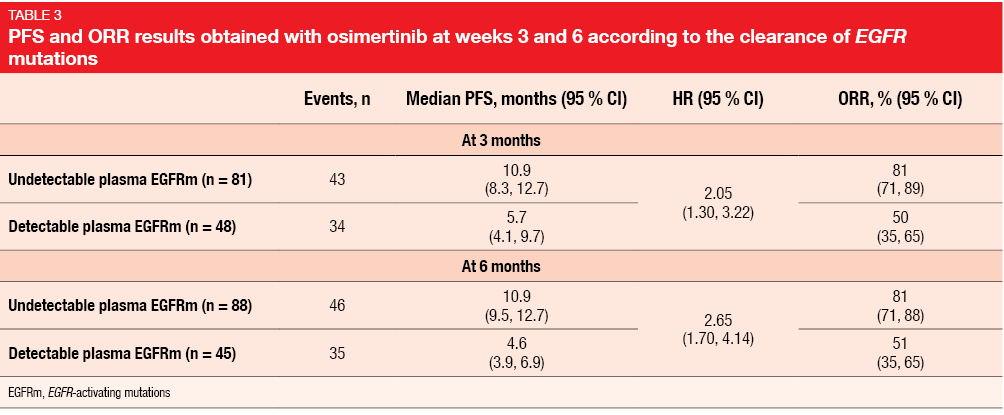
Also, early dynamic changes of plasma EGFR mutations might predict PFS in patients receiving treatment for T790M-positive NSCLC, as continued presence of circulating tumour DNA (ctDNA) for EGFR mutations at weeks 3 and 6 was associated with less favourable PFS and ORR results (Table 3). Thus, patients with T790M-positive NSCLC who might experience sub-optimal clinical outcomes could be identified as early as 3 weeks after initiation of osimertinib treatment. Monitoring of ctDNA for EGFR mutations might allow for modification of treatment with the aim of outcome optimisation.
Another potential early marker for the prediction of responses to osimertinib is 18F-fluorodeoxyglucose (FDG) PET. Yoon et al. conducted a prospective, open-label, single-centre pilot study in 25 patients who had shown disease progression on first-generation EGFR TKI treatment [22]. ORR was 76 %, with the metabolic response (defined as ≥ 20 % decrease of ΔSUVmax) being significantly related to median PFS and ORR. Osimertinib showed anti-tumour activity even in patients harbouring no T790M mutation.
Ramucirumab as a combination partner
The ongoing phase I JVDL trial is assessing the combination of osimertinib with the monoclonal anti-VEGFR-2 antibody ramucirumab in EGFR-mutant, T790M-positive NSCLC after progression on first-line EGFR TKI treatment. In their analysis of 25 patients, Planchard et al. showed that the safety profile of the combination was consistent with the safety profile of each drug as monotherapy, with no additive toxicities [23]. Hypertension, diarrhoea, stomatitis, rash, and thrombocytopenia constituted the most common AEs. Discontinuation due to AEs occurred only in 4 %.
Furthermore, the results suggest encouraging anti-tumour activity. Complete or partial responses were achieved in 76 %, and disease control in 92 %. Median DOR had not been reached at the time of the analysis, which also applied to median PFS. At 12 months, 57.5 % of patients were alive and progression-free.
New kid on the block: nazartinib
Nazartinib is an investigational third-generation, irreversible EGFR TKI selective for activating EGFR mutations and T790M mutations while sparing wild-type EGFR. According to the preliminary phase II results of a multicentre, open-label phase I/II first-in-human study, nazartinib shows a tolerable safety profile and promising efficacy in treatment-naïve patients with EGFR-mutant, stage IIIB/IV NSCLC [24]. Forty percent of patients had brain metastases at screening.
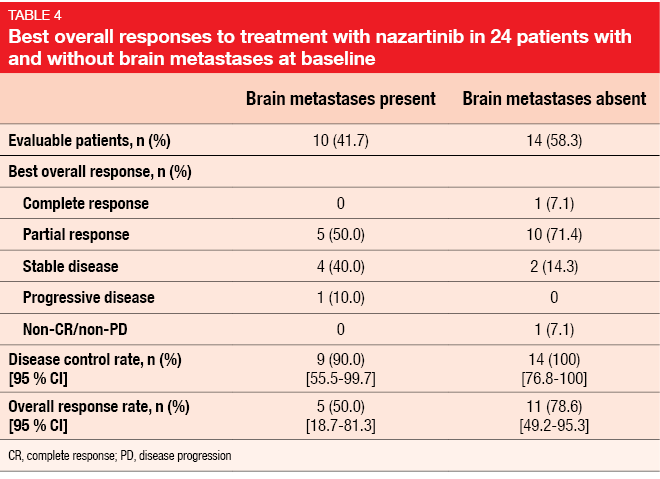
Nazartinib was well tolerated, with the majority of AEs being grade 1 or 2. Overall, the safety profile appeared favourable in terms of all typical toxicities, such as diarrhoea, acneiform rash, dry skin, stomatitis, and paronychia. Maculopapular rash was the most frequent AE, but proved manageable. Despite the high proportion of patients with brain lesions at baseline, the new TKI elicited an ORR of 66.7 % according to the blinded independent review committee. Disease control occurred in 95.8 %. The majority of patients experienced reductions in the size of target lesions. Nazartinib was effective in patients both with and without brain metastases (Table 4). PFS and DOR data were still immature at the data cut-off. A phase III study investigating nazartinib in the treatment-naïve setting is projected to start in July 2018.
REFERENCES
- Furuya N et al., Phase III study comparing bevacizumab plus erlotinib to erlotinib in patients with untreated NSCLC harboring activating EGFR mutations: NEJ026. J Clin Oncol 36, 2018 (suppl; abstr 9006)
- Seto T et al., Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014; 15(11): 1236-1244
- Maemondo M et al., Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362(25): 2380-2388
- Sugawara S et al., Randomized phase II study of concurrent versus sequential alternating gefitinib and chemotherapy in previously untreated non-small cell lung cancer with sensitive EGFR mutations: NEJ005/TCOG0902. Ann Oncol 2015; 26(5): 888-894
- Nakamura A et al., Phase III study comparing gefitinib monotherapy to combination therapy with gefitinib, carboplatin, and pemetrexed for untreated patients with advanced non-small cell lung cancer with EGFR mutations (NEJ009). J Clin Oncol 36, 2018 (suppl; abstr 9005)
- Wu YL et al., Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017; 18(11): 1454-1466
- Mok TS et al., Improvement in overall survival in a randomized study comparing dacomitinib with gefitinib in patients with advanced non-small cell lung cancer harboring EGFR-activating mutations. J Clin Oncol 36, 2018 (suppl; abstr 9004)
- Tan DSW, Shifting Sands: improving on first-generation EGFR TKIs. ASCO 2018 Congress, Discussion of abstracts 9004 and 9005
- Park K et al., Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016; 17(5): 577-589
- Halmos B et al., Real-world dose adjustment study of first-line afatinib in pts with EGFR mutation-positive (EGFRm+) advanced NSCLC. J Clin Oncol 36, 2018 (suppl; abstr e21060)
- Tanaka H et al., Real world study of afatinib in first-line or re-challenge setting for patients with EGFR mutant non-small cell lung cancer. J Clin Oncol 36, 2018 (suppl; abstr e211713)
- Domine M et al., Clinical activity of afatinib in a cohort of patients with lung adenocarcinoma harbouring uncommon EGFR mutations: A Spanish retrospective multicentre study. J Clin Oncol 36, 2018 (suppl; abstr e21028)
- Sequist LV et al., The ASCENT trial: a phase II study of neoadjuvant afatinib, chemoradiation and surgery for stage III EGFR mutation-positive NSCLC. J Clin Oncol 36, 2018 (suppl; abstr 8544)
- Paz-Ares L et al., PACIFIC: a double-blind, placebo-controlled Phase III study of durvalumab after chemoradiation therapy (CRT) in patients with stage III, locally advanced, unresectable NSCLC, ESMO 2017, abstract LBA1_PR
- Offin M et al., Tumor mutation burden and efficacy of EGFR-tyrosine kinase inhibitors in patients with EGFR-mutant lung cancers. J Clin Oncol 36, 2018 (suppl; abstr 9042)
- Mok TS et al., Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017; 376: 629-640
- Soria JC et al., Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018; 378(2):113-125
- Le X et al., Landscape of EGFR-dependent and -independent resistance mechanisms to osimertinib in EGFR-mutant NSCLC. J Clin Oncol 36, 2018 (suppl; abstr 9087)
- Zhang Y et al., Landscape of osimertinib resistant mutations between the two common subtypes of EGFR 19del or L858R in NSCLC. J Clin Oncol 36, 2018 (suppl; abstr 12108)
- Thress KS et al., Complete clearance of plasma EGFR mutations as a predictor of outcome on osimertinib in the AURA trial. J Clin Oncol 35, 2017 (suppl; abstr 9018)
- Shepherd FA et al., Early clearance of plasma EGFR mutations as a predictor of response to osimertinib in the AURA3 trial. J Clin Oncol 36, 2018 (suppl; abstr 9027)
- Yoon S et al., The role of FDG-PET during osimertinib treatment to predict the responsiveness of tumor early in patients with stage IV non-small cell lung cancer: A pilot study. J Clin Oncol 36, 2018 (suppl; abstr e21150)
- Planchard D et al., Efficacy and safety results of ramucirumab in combination with osimertinib in advanced T790M-positive EGFR-mutant NSCLC. J Clin Oncol 36, 2018 (suppl; abstr 9053)
- Kim D-W et al., Preliminary phase II results of a multicenter, open-label study of nazartinib (EGF816) in adult patients with treatment-naïve, EGFR-mutant NSCLC. J Clin Oncol 36, 2018 (suppl; abstr 9094)
More posts
Comprehensive sequencing of plasma cell-free DNA permits non-invasive cancer detection
Early detection of lung cancer is a highly unmet medical need. Even though low-dose computed tomography (LDCT) has been shown to improve lung cancer mortality in high-risk individuals, the rate of clinical adoption remains low at 1.9 %. Cell-free DNA (cfDNA) testing might substitute LDCT as a screening tool, according to preliminary results of the Circulating Cell-free Genome Atlas (CCGA) Study presented at the ASCO Congress.
Recent benchmarks in the management of small-cell tumours
Extensive-disease small-cell lung cancer (ED-SCLC) is highly responsive to first-line therapy, but early relapses commonly occur, and prognosis is poor. To date, no biomarker-driven therapies have been established. Based on the involvement of the immune system in the pathophysiology of SCLC and the high mutational burden of this disease, immunotherapy has potential as a novel treatment option.
ALK-positive disease: pushing the borders of treatability
Standard treatment for patients with ALK-positive, advanced NSCLC includes the first-generation ALK inhibitor crizotinib and, more recently, second-generation ALK TKIs such as ceritinib and alectinib. The global, phase III ALEX trial tested the highly selective, CNS-active ALK inhibitor alectinib as first-line agent compared to crizotinib in patients with stage IIIB/IV ALK-positive NSCLC. Asymptomatic brain metastases were allowed in this study.
Interview with Barbara Melosky: “The sequencing question remains”
Afatinib has been licensed for the second-line treatment of patients with squamous-cell carcinoma of the lung. A combination trial is ongoing that is testing afatinib plus pembrolizumab. What can we expect from this regimen?
New data on EGFR-directed TKIs across 3 generations
EGFR TKI treatment has become a standard first-line strategy for patients with advanced, EGFR-mutation–positive NSCLC. Established agents include the first-generation drugs gefitinib and erlotinib, the second-generation agents afatinib and dacomitinib, and the third-generation TKI osimertinib. Combinations of EGFR TKIs with other drug classes might lead to outcome optimisation, for instance the additional administration of anti-angiogenic drugs, such as bevacizumab and ramucirumab.
Immune checkpoint blockade: determinants of treatment success
Various clinical factors beyond PD-L1 expression have been explored as predictors of response to immune checkpoint inhibition. Specifically, analyses have associated lack of tobacco exposure with diminished responsiveness to PD-1 pathway blockade in NSCLC. One possible explanation for this is that lung cancers arising in never or minimal smokers generally show low TMB.