How does checkpoint inhibition perform in the setting of oncogene-driven lung cancer?
Impact of various aberrations
Retrospective analyses have demonstrated limited activity of immune checkpoint inhibitors (CPIs) in patients with actionable oncogenic driver mutations [1, 2]. In similar vein, the randomized controlled IMpower150 and IMpower130 studies revealed no survival benefit of adding CPIs to platinum doublets in patients who harbored EGFR and ALK aberrations [3, 4].
The retrospective study reported by Kelly et al. was conducted to describe PFS and other endpoints with chemotherapy plus CPIs versus chemotherapy alone in the setting of oncogene-driven NSCLC [5]. The patients included had at least one driver mutation (EGFR, ALK, ROS1, MET, RET, KRAS, HER2, NTRK). Between January 2018 and December 2019, they received platinum-based doublet regimens with or without checkpoint inhibition at the NCI-designated University of California Cancer Centers.
Overall, 147 patients were included in the analysis. EGFR mutations constituted the most common driver alterations (49.7 %), followed by KRAS mutations (36.7 %) and ALK fusions (6.8 %). Two percent of patients had MET mutations. HER2 mutations, RET fusions and ROS1 fusions were present in 1.4 % each, and BRAF mutations in 0.7 %. PD-L1 expression of 1-49 % and ≥ 50 % was found in 25.2 % and 19.7 %, respectively. Thirty percent of tumors did not express PD-L1, and in 24.5 %, the PD-L1 status was unknown.
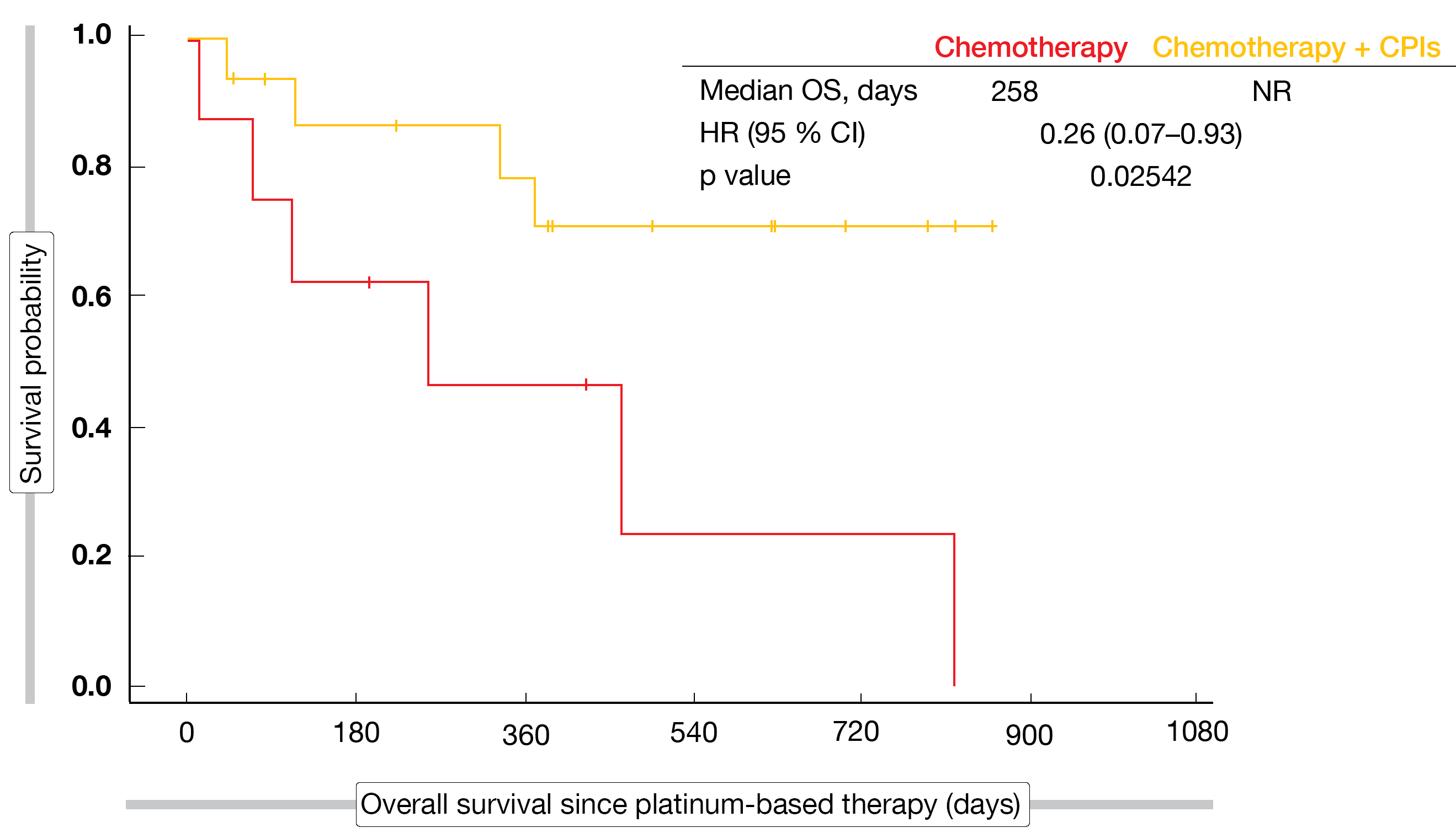
Chemotherapy plus immunotherapy, as compared to chemotherapy only, did not confer any significant PFS or OS benefits in either group except for the small cohort with KRASG12C-mutated tumors. In this population, median PFS was 249 vs. 93 days with chemotherapy plus CPIs vs. chemotherapy (HR, 0.31; p = 0.01415). Median OS had not been reached in the immunotherapy-treated patients, while it was 258 days for chemotherapy only (HR, 0.26; p = 0.02542; Figure). The analysis identified no new safety concerns.
Moreover, PFS and OS were compared in additional cohorts. These comprised never smokers, current/former smokers, patients on first-line therapy and patients on second- or later-line therapy. In none of these, significant PFS or OS differences were observed for chemotherapy plus immunotherapy vs. chemotherapy. This was also true for the entire cohort where 71 and 76 patients received the combined strategy and chemotherapy alone, respectively. Here, risk reductions for PFS and OS were 7 % (HR, 0.93; p = 0.69832) and 26 % (HR, 0.74; p = 0.18754), respectively.
Figure: Overall survival for chemotherapy plus CPIs vs. chemotherapy in patients with KRASG12C mutations
Anti-PD-(L)1 agents & KRAS status
While phase III trials on agents targeting KRAS in patients with KRAS-mutant NSCLC are ongoing, the clinical efficacy of anti-PD-(L)1 therapies in this subgroup remains a topic of debate. Therefore, Landre et al. conducted a meta-analysis of randomized studies investigating first-line or second-line anti-PD-(L)1 antibodies with or without chemotherapy vs. chemotherapy alone in patients with advanced KRAS-mutant NSCLC [6]. Six trials assessing pembrolizumab, atezolizumab or nivolumab in a total of 4,809 patients were included in the analysis. The proportions of patients with KRAS-mutant disease enrolled in these studies ranged between 23 % and 38 %.
Anti-PD-(L)1 therapies with or without chemotherapy were shown to achieve longer OS and PFS than chemotherapy alone in both KRAS-mutant and KRAS-wildtype patients, with an even greater benefit for the mutated cohort. In this population, the experimental treatment, as compared to chemotherapy, led to a 41 % reduction in mortality risk (HR, 0.59; p < 0.00001) and a 42 % reduction in the risk of progression and death (HR, 0.58; p = 0.0003). OS benefits were observed in both first-line and second-line trials. In the KRAS-wildtype population, patients treated with immunotherapy derived a 13 % OS benefit (HR, 0.87). The comparison across the two populations showed that OS for patients with KRAS mutation was significantly longer than for patients with KRAS wildtype (p = 0.001).
G12C mutations vs. non-G12C mutations
Another analysis related to the efficacy of first-line chemo-immunotherapy regimens in patients with KRAS-mutant advanced/metastatic lung cancer treated at the Memorial Sloan Kettering Cancer Center and Dana-Farber Cancer Institute [7]. This group comprised 69 and 93 patients with G12C and non-G12C KRAS mutations, respectively. Less than half of tumors in both cohorts were PD-L1–positive. PD-L1 expression of 1-49 % was present in 31 % and 35 % of patients with G12C mutations and non-G12C mutations, respectively; for PD-L1 expression of 50-100 %, this was 12 % and 11 %, respectively.
Patients with G12C mutations, as compared to those with non-G12C mutations, derived greater benefits from chemo-immunotherapy in terms of PFS (6.9 vs. 6.0 months; p = 0.04) and OS (21.3 vs. 14.3 months; p = 0.07). Furthermore, the researchers evaluated the impact of STK11 and KEAP1 co-mutations within the G12C-mutated group. Fifty-six percent of patients had neither mutation, while STK11 was mutated in 15 %, KEAP1 in 6 %, and both in 23 %. Patients with STK11 and KEAP1 wildtype were shown to do considerably better with chemo-immunotherapy than those with STK11 and/or KEAP1 mutations. Median PFS for these two groups was 15.8 vs. 5.6 months (p = 0.03). Likewise, wildtype patients developed complete or partial responses more frequently than those harboring mutations, although this difference was not significant (p = 0.11).
In addition to the co-mutation status, PD-L1 expression affected the outcomes to chemo-immunotherapy in the G12C-mutated group. Objective responses were more likely in the presence of PD-L1 positivity compared to PD-L1 negativity, and median PFS was longer (10.7 vs. 6.8 months), although neither of these differences was significant. In their conclusions, the authors noted that co-mutation pattern and PD-L1 expression status might identify patients with KRAS-mutated lung cancer most in need of alternative first-line therapies such as KRASG12C inhibitors.
Real-world data for CPIs & STK11 co-mutation
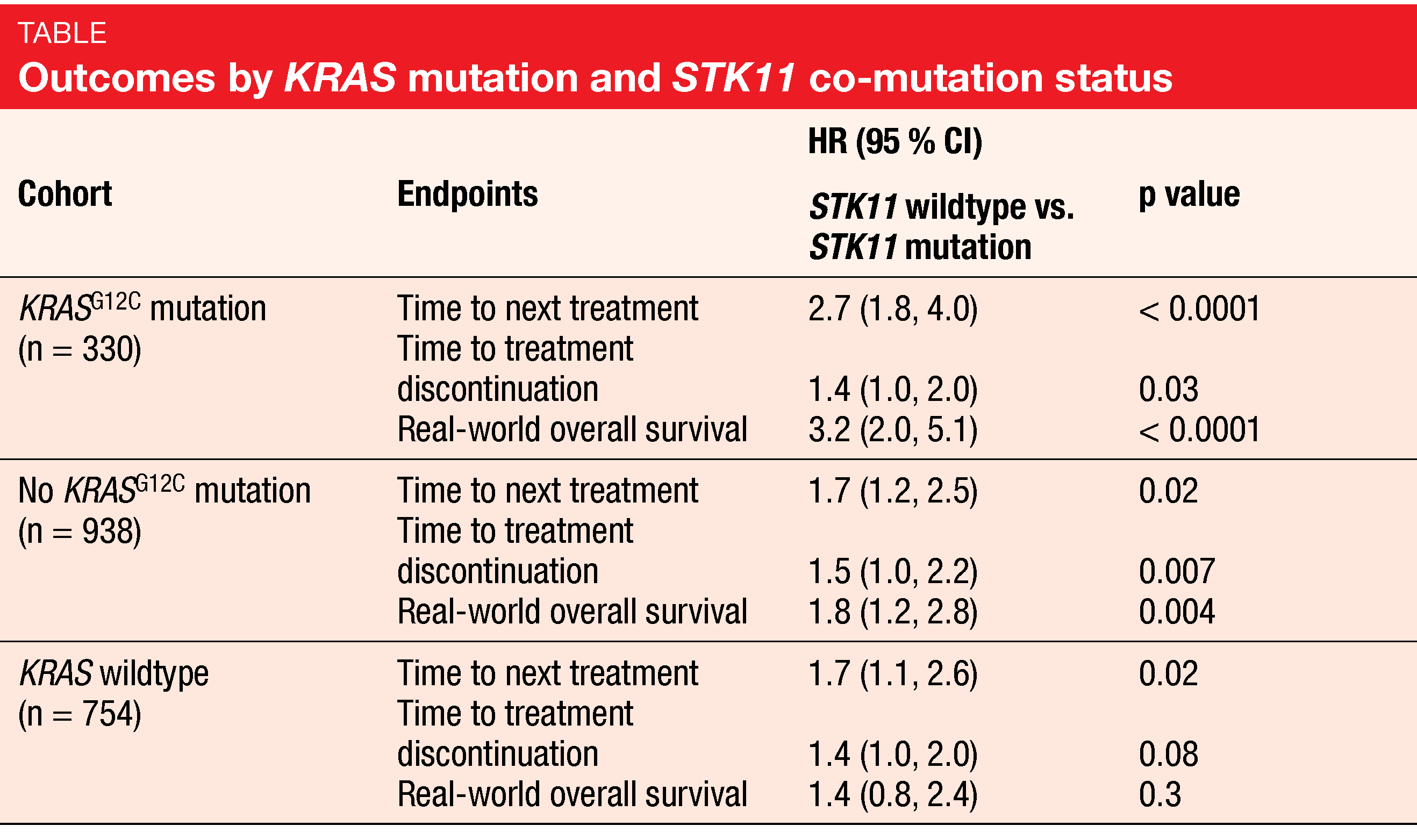
These findings were corroborated by real-world evidence reported at ASCO 2021 that assessed the impact of co-occurring STK11 mutations on outcomes in patients with KRASG12C-mutant adenocarcinoma of the lung treated with a first-line CPI-containing regimen. Heist et al. conducted a retrospective observational real-world study based on Guardant INFORM, which is a nationally representative US healthcare claims clinical-genomic dataset [8]. The researchers identified 330 and 938 patients with and without KRASG12C mutations, respectively. In the KRASG12C-mutated cohort, co-occurring mutations in the STK11 gene were present in 21 %. The matched cohort without KRASG12C mutation contained patients with other KRAS mutations, as well as patients with KRAS wildtype; the latter represented 80 % of the group. STK11 mutations were found in 9 % of those with other KRAS mutations and in 6 % of those with KRAS wildtype.
According to this analysis, KRASG12C and STK11 co-mutations are associated with poor outcomes in patients treated with first-line immunotherapy. Time to next treatment (TTNT) was over four times shorter in the co-mutated group than in KRASG12C-mutated patients without STK11 mutations (224 vs. 975 days; HR, 2.7; p < 0.0001; Table). Also, time to treatment discontinuation (TTD) was seriously reduced (172 vs. 232 days; HR, 1.4; p = 0.03), and real-world OS (rwOS) was increased by a factor of 3.2 (p < 0.0001).
Smaller differences across patients with and without co-mutations were noted for the matched no KRASG12C cohort and the KRAS wildtype cohort (Table). Patients with other KRAS mutations who harbored STK11 co-mutation had significantly shorter TTNT, TTD and rwOS than those without STK11 mutations, although the adjusted HRs of TTNT and rwOS were lower than the HRs for the KRASG12C cohort. In the matched KRAS wildtype group, the differences for TTD and rwOS did not reach statistical significance. The authors summarized that these inferior outcomes indicate the high need for effective targeted and/or combination therapies in NSCLC patients with co-occurring KRASG12C and STK11 mutations.
REFERENCES
- Gainor JF et al., EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res 2016; 22(18): 4585-4593
- Mazieres J et al., Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019; 30(8): 1321-1328
- Reck M et al., Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019; 7(5): 387-401
- West H et al., Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20(7): 924-937
- Kelly K et al., Role of chemotherapy plus immune checkpoint inhibition in oncogenic driven lung cancer: a University of California Lung Cancer Consortium retrospective study. J Clin Oncol 39, 2021 (suppl 15; abstr 9059)
- Landre T et al., Anti-PD-(L)1 for KRAS-mutant advanced non-small-cell lung cancers: a meta-analysis of randomized, controlled trials. J Clin Oncol 39, 2021 (suppl 15; abstr 9025)
- Arbour KC et al., Chemo-immunotherapy outcomes of KRAS-G12C mutant lung cancer compared to other molecular subtypes of KRAS-mutant lung cancer. J Clin Oncol 39, 2021 (suppl 15; abstr 9088)
- Heist R et al., Impact of STK11 mutation on first-line immune checkpoint inhibitor outcomes in a real world KRAS G12C mutant lung adenocarcinoma cohort. J Clin Oncol 39, 2021 (suppl 15; abstr 9106)
- Jones C et al., Impact of immune checkpoint inhibitor and EGFR tyrosine kinase inhibitor sequence on time to treatment failure among EGFR+ NSCLC treated in a community-based cancer research network. J Clin Oncol 39, 2021 (suppl 15; abstr 9099)
© 2021 Springer-Verlag GmbH, Impressum