Early-stage NSCLC: perioperative strategies and approaches in the unresectable
Neoadjuvant chemotherapy has been shown to significantly prolong overall survival (OS) in resectable non–small-cell lung cancer (NSCLC), although the absolute 5-year survival is improved by as little as 5 % [1]. Similarly, pathological complete responses (pCR) are infrequently achieved; 15 trials investigating preoperative chemotherapy revealed an overall median rate of 4 % [2]. A promising approach to enhance treatment success involves the addition of immunotherapy to platinum-based neoadjuvant chemotherapy. The phase III CheckMate 816 study assessing nivolumab plus chemotherapy compared to chemotherapy alone revealed significant benefits regarding pCR (24.0 % vs. 2.2 %; p < 0.001) and event-free survival (EFS; 31.6 vs. 20.8 months; p = 0.005) in patients with stage IB-IIIA NSCLC [3].
NADIM II
In the setting of potentially resectable stage IIIA-B disease, the randomized, open-label, phase II NADIM II trial confirmed the superiority of neoadjuvant nivolumab plus chemotherapy. Patients in the experimental arm (n = 57) received nivolumab 360 mg in addition to paclitaxel and carboplatin for 3 cycles, while those in the control arm (n = 29) were treated with chemotherapy alone. After surgery, adjuvant nivolumab 480 mg Q4W was administered for 6 months in the experimental arm. The control patients were observed only. At ASCO 2022, Provencio et al. reported the primary endpoint results of pCR and other outcomes [4].
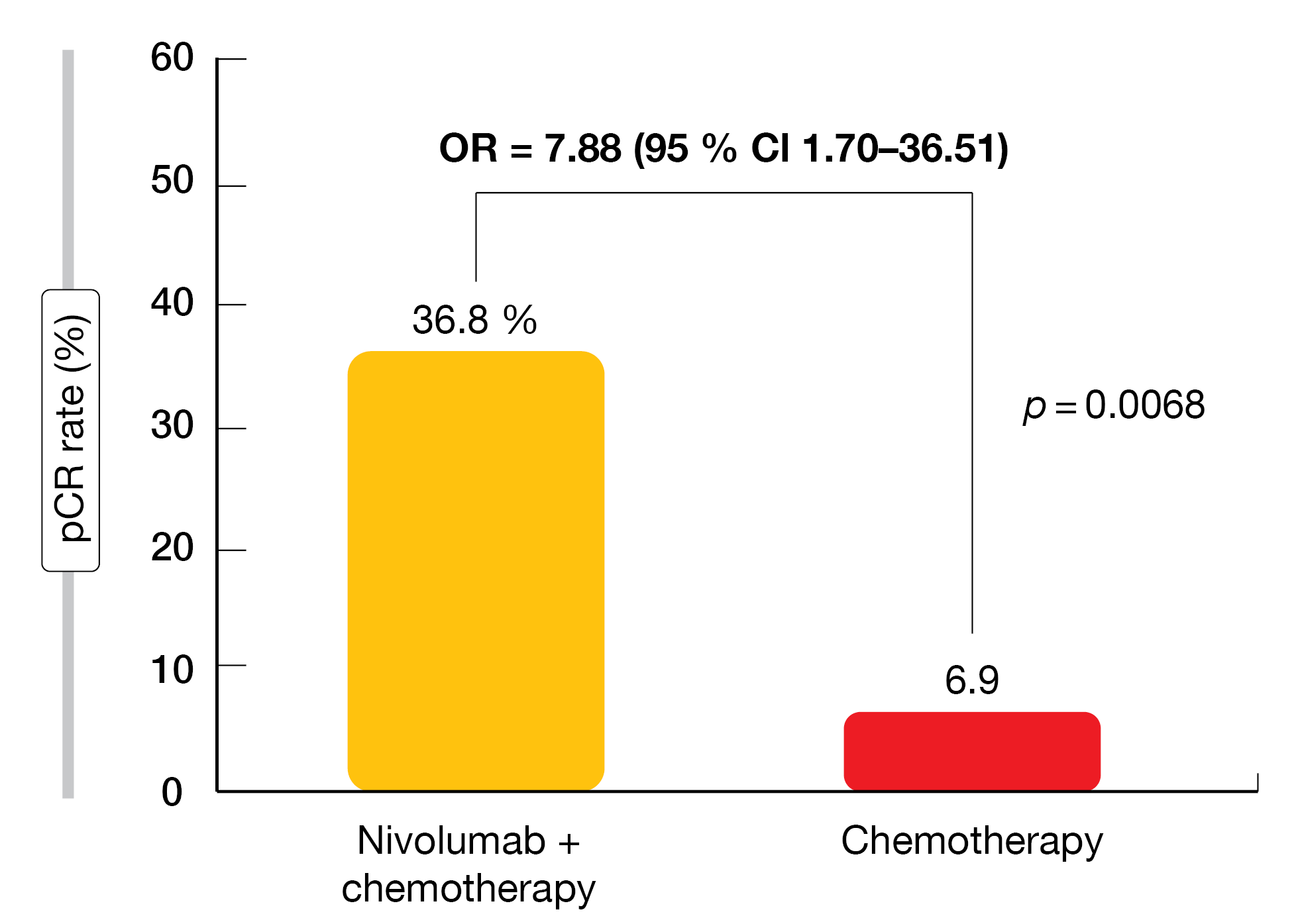
Indeed, the pCR was significantly increased with immunochemotherapy (36.8 % vs. 6.9 %; OR, 7.88; p = 0.0068; Figure 1), which translated into a number needed to treat of 3.34. Similarly, the combined approach induced significant benefits regarding the major pathological response (MPR) rate (52.6 % vs. 13.8 %; OR, 6.94; p = 0.0012) and the overall response rate (ORR; 75.4 % vs. 48.2 %; OR, 3.29; p = 0.023). The addition of nivolumab did not impede the feasibility of surgery; on the contrary, definitive surgery was significantly more common in the experimental arm (93.0 % vs. 69.0 %; OR, 5.96; p = 0.00807).
Despite the addition of the PD-L1 inhibitor, a tolerable safety profile was maintained, with a moderate increase in grade 3/4 adverse events (AEs; 25 % vs. 10.3 %). Grade 4 events occurred only in 2 patients in the experimental arm. None of the study participants died due to AEs. The PD-L1 tumor proportion score (TPS) was shown to have a significant predictive value for pCR, with pCR rates rising across increasing TPS categories (p = 0.014).
Figure 1: Superior pCR rate with neoadjuvant nivolumab plus chemotherapy vs. chemotherapy in NADIM II
pCR correlates with EFS in CheckMate 816
While studies have shown an association of pathological response and survival with neoadjuvant chemotherapy in resectable NSCLC and other cancers [2, 5, 6], a similar correlation with neoadjuvant immunotherapy has not been rigorously studied. At ASCO 2022, Provencio-Pulla et al. presented a post-hoc analysis on the association between pathological regression and EFS in patients who underwent surgery in CheckMate 816 and had pathologically evaluable samples (i.e., the path-evaluable population) [7]. EFS was assessed according to pCR or MPR in the primary tumor, as well as by depth of pathological regression, measured by percentage of residual viable tumor (RVT) in the primary tumor. The path-evaluable population group included 141 and 126 patients in the experimental and control arms, respectively. This analysis provides the first in-depth assessment of pathological regression and EFS in a phase III trial with neoadjuvant immunotherapy.
EFS was improved with both nivolumab/chemotherapy and chemotherapy in patients with pCR or MPR in the primary tumor relative to those without. In the combination arm, EFS improvement in patients who had pCR was achieved regardless of baseline stage of disease or tumor PD-L1 expression. No corresponding subgroup analyses were conducted for the chemotherapy-only arm due to small sample sizes. Nivolumab-treated patients with deeper pathological regression in the primary tumor appeared to have better EFS outcomes at 2 years, with the RVT reduction as a continuous variable being predictive of 2-year EFS. This did not apply to the chemotherapy-only arm.
In the path-evaluable population, the incidence of treatment-related AEs in the combination arm was similar in patients with or without pCR/MPR. This finding was consistent with the observation in all treated patients. Overall, these results from CheckMate 816, along with previously reported data, continue to support pathological response as an early indicator of EFS benefit with neoadjuvant nivolumab plus chemotherapy.
Neoadjuvant CRT compared to CT
Stage III NSCLC is a heterogeneous disease, making this subgroup of patients a challenging population. Stage III N2, which is characterized by ipsilateral mediastinal or subcarinal lymph node metastasis, represents the most advanced stage that still allows for a curative approach. If the disease is deemed resectable, neoadjuvant chemoradiotherapy (CRT) or chemotherapy (CT) precedes surgery, with the main goal of CRT being mediastinal downstaging from N2 to N1 or N0.
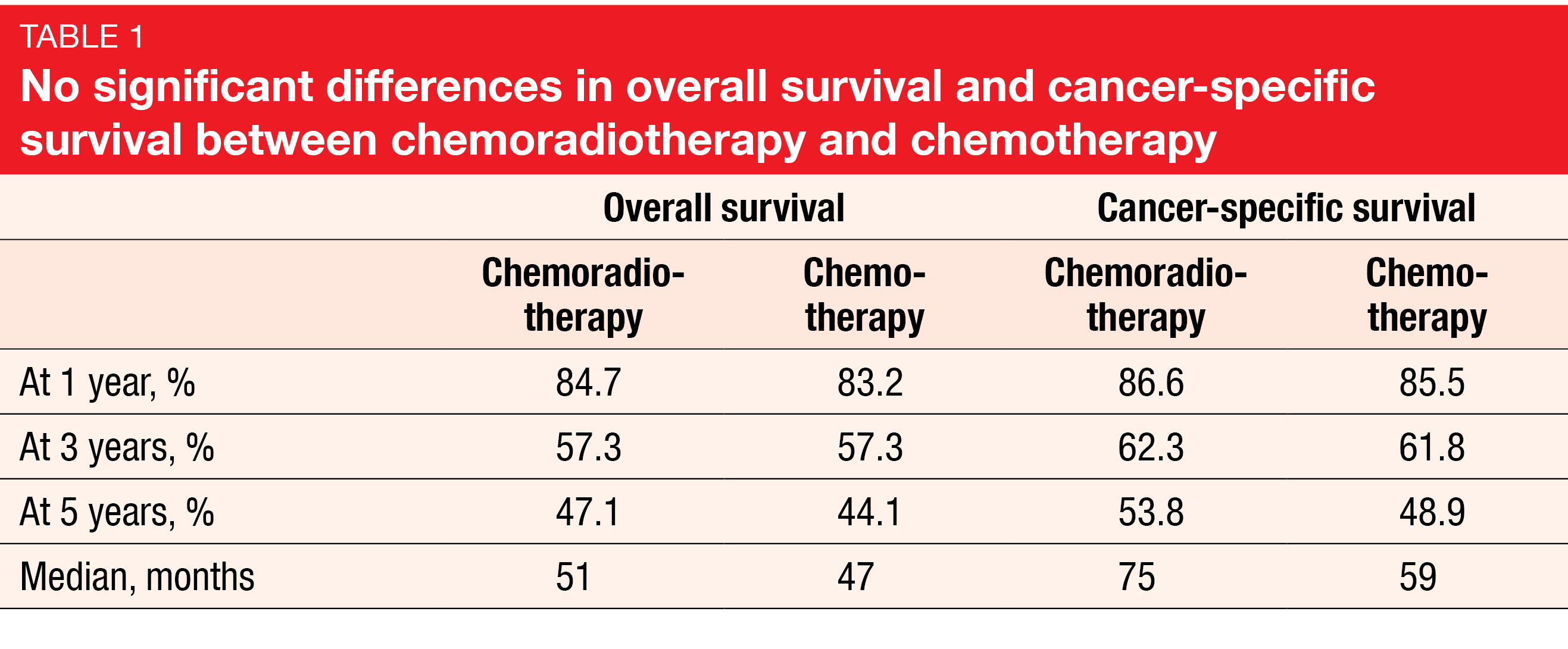
Several clinical trials have demonstrated no added benefit of radiotherapy to neoadjuvant CT in early-stage disease [8-12]. In view of these observations, a real-world study was conducted based on the SEER database to identify patients with stage III N2 M0 disease who had received either CRT or CT prior to surgery from 2004 to 2015 [13]. The researchers obtained data for 1,175 patients, with 799 (68.0 %) and 376 (32.0 %) having been treated with CRT and CT, respectively. OS and cancer-specific survival (CSS) constituted the primary outcomes. This is the largest retrospective cohort in this field to date and the first study to address CSS.
Overall, the findings indicated no survival benefit of adding RT to CT in the neoadjuvant setting in N2 disease. The OS rates at 1 year, 3 years, and 5 years were almost identical for CRT vs. CT, and the median OS differed only by 4 months in favor of CRT (Table 1). For CSS, the results were similar despite a higher difference regarding median CSS. Nevertheless, neither endpoint showed statistically significant superiority of the combined approach. This also applied to the multivariate analysis and the inverse probability treatment weighting analysis conducted to eliminate the effect of the non-randomization bias. Significant prognostic factors for adverse OS and CSS were higher T stage, non-squamous histology, higher lymph node ratio, tumor location in the lower lobes, and pneumonectomy. The authors concluded that the combined treatment could be more harmful than useful. They recommended comparing CSS in stage IIIB disease, the assessment of different types of N2 disease, and consideration of chemoimmunotherapy modalities in neoadjuvant settings.
Three vs. 2 neoadjuvant cycles
Clinical trials investigating neoadjuvant chemoimmunotherapy generally used 2-4 cycles [3, 14-16], although there is no consensus regarding the optimal treatment duration. The phase II neoSCORE trial tested 2 cycles vs. 3 cycles of neoadjuvant treatment with the anti-PD-1 antibody sintilimab plus chemotherapy in the setting of resectable stage IB-IIIA NSCLC. Surgery was performed within the 4th week after the last dose and was followed by adjuvant chemotherapy for 1 or 2 cycles and either maintenance treatment with sintilimab for up to 1 year, or follow-up alone. The MPR rate was defined as the primary endpoint. NeoSCORE is the first randomized study comparing different treatment periods of immunochemotherapy in the neoadjuvant setting.
The analysis reported at ASCO 2022 included approximately 30 patients in each treatment arm [17]. Three cycles, as compared to 2, gave rise to a numerically higher MPR rate, translating into a 14.5 % increase (41.4 % vs. 26.9 %; p = 0.260). The absence of statistical significance is potentially due to the narrow cycle difference and the small sample size. Moreover, one additional cycle increased the pCR rate by 4.9 % (24.1 % vs. 19.2 %; p = 0.660) and the radiologically assessed ORR by 5.2 % (55.2 % vs. 50.0 %; p = 0.701). Most subgroups fared better with 3 cycles than with 2, particularly the group with clinical stage IIIA.
According to the assessment by histology, patients with squamous NSCLC achieved impressively higher MPR rates than those with the non-squamous subtype in both treatment arms; these differences were statistically significant for both 3 cycles (60 % vs. 21.4 %; p = 0.035) and 2 cycles (43.8 % vs. 0 %; p = 0.023). Across the arms, the MPR rates amounted to 51.6 % vs. 12.5 % for patients with squamous vs. non-squamous histology (p = 0.002). Similar trends were observed with respect to the pCR rate (29.0 % vs. 12.5 %; p = 0.141) and the ORR (61.3 % vs. 41.7 %; p = 0.148). The PD-L1 expression had modest predictive value for the pathological response, as patients with PD-L1 ≥ 45 % experienced improved tumor responses (p = 0.003 and 0.024 for pathological regression and change from baseline, respectively).
Planned surgery was conducted in 93.5 % vs. 89.7 % of patients in the 3-cycle vs. 2-cycle arms. No increases of surgical risk (e.g., duration of surgery, intraoperative blood loss) or postoperative complications (e.g., recurrent laryngeal nerve paresis, atrial fibrillation, pleural effusion requiring drainage) were observed with 3 treatment cycles. Treatment-related hematological and non-hematological AEs did not increase in the experimental arm. Overall, these data suggest that a higher number of cycles of neoadjuvant chemoimmunotherapy provides an improved MPR rate for patients with resectable NSCLC, especially in the squamous subtype.
Intraoperative quality metrics
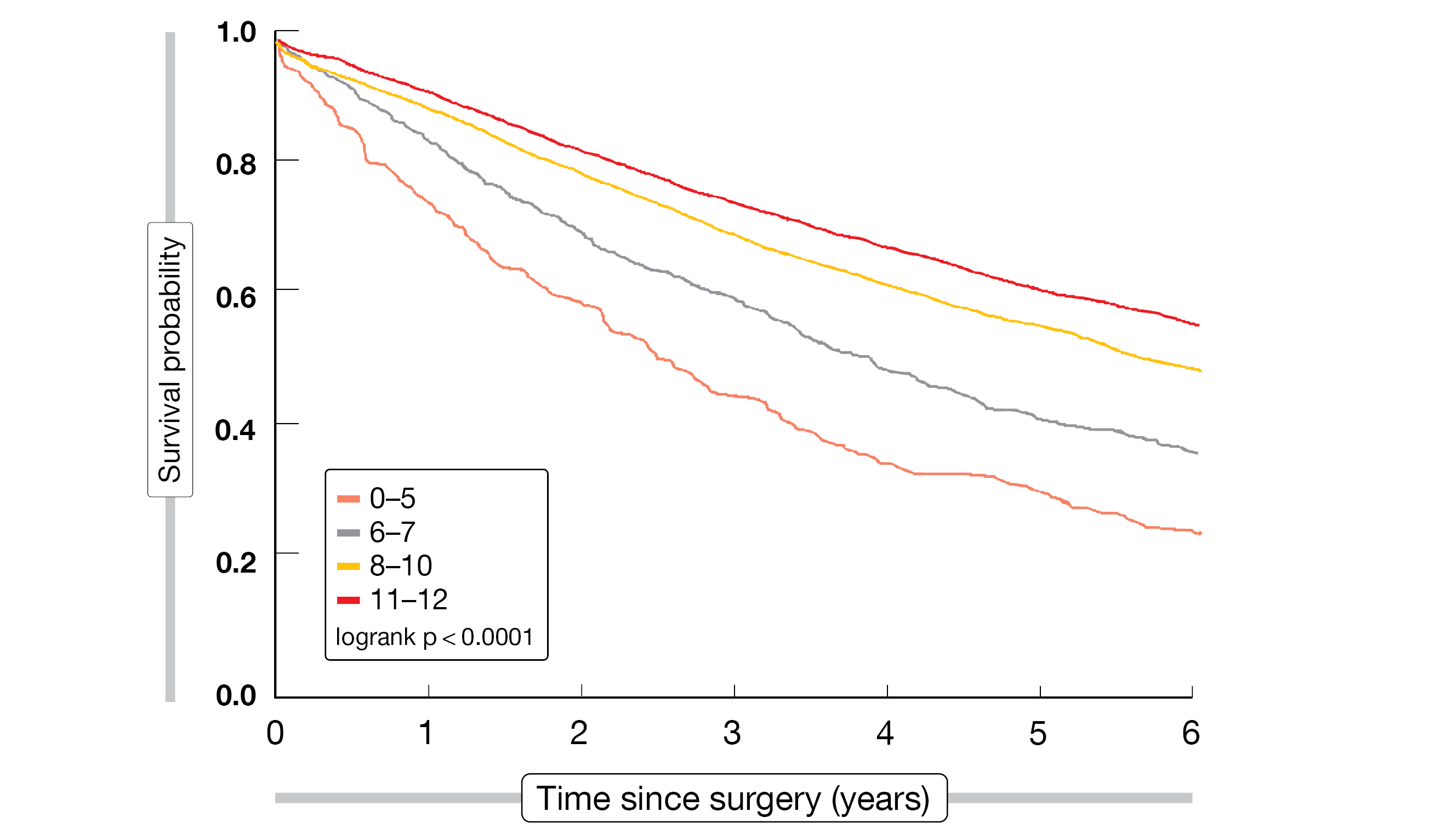
Heiden et al. sought to develop a practical surgical quality score for patients who undergo definitive surgical treatment for stage I NSCLC [18]. The modifiable quality metrics used for this purpose have been identified previously and included timely surgery (i.e., surgery within 12 weeks of radiographic NSCLC diagnosis), anatomic resection via lobectomy or segmentectomy, minimally invasive approach using video-assisted thoracoscopic surgery or a robotic approach, adequate nodal sampling (i.e., ≥ 10 lymph nodes according to ACS CoC standards), and negative (R0) surgical margin. Data of 9,628 veterans with clinical stage I NSCLC from the Veterans’ Health Administration Corporate Data Warehouse who underwent surgery between 2006 and 2016 were analyzed. The researchers developed a VA Lung Cancer Operative quality (VALCAN-O) score ranging from 0 to 12 points, with the highest scores representing the best surgical quality. After division of the patients into risk categories based on their scores, median OS (Figure 2) and recurrence-free survival were demonstrated to differ substantially. These findings were validated in an external cohort of > 100,000 patients from the National Cancer Database (2010-2016). Survival probability again differed between the score categories in a clear-cut manner.
Moreover, the researchers assessed geographical trends in surgical quality across the USA from 2006 to 2019. Here, the VALCAN-O scores improved substantially over the study period in most regions; however, significant regional variation was observed even up until 2019, particularly in the Midwest and the Eastern US. Adherence to most of the quality metrics generally improved throughout the study period. Minimally invasive approaches showed a dramatic rise over time. Adequate nodal sampling also increased, while delayed surgery decreased. Conversely, the proportion of lobectomy is decreasing, whereas segmentectomy increased slightly. As the authors noted, adherence to modifiable surgical quality metrics is associated with dramatically improved long-term, cancer-specific outcomes. The VALCAN-O quality score can serve as a benchmark of surgical quality in lung cancer, thereby standardizing and improving outcomes among patients with early-stage lung cancer undergoing curative-intent resection.
Figure 2: Overall survival outcomes after surgery according to VALCAN-O score categories
Adjuvant pembrolizumab: impact of variables on DFS
The global, randomized, triple-blind, phase III PEARLS/KEYNOTE-091 trial tested pembrolizumab vs. placebo as adjuvant treatment in patients with stage IB (tumor size ≥ 4 cm) to IIIA NSCLC following complete surgical resection with negative margins and, when recommended per local guidelines, adjuvant chemotherapy for ≤ 4 cycles. At the time of the second interim analysis, pembrolizumab treatment significantly improved disease-free survival (DFS) in the overall population (53.6 vs. 42.0 months; HR, 0.76; p = 0.0014) [19]. In the group with PD-L1 TPS ≥ 50 %, the DFS difference did not reach statistical significance (HR, 0.82; p = 0.14). Immature OS data suggested a trend towards improvement in the experimental arm. The analysis of the PEARLS/KEYNOTE-091 study reported at ASCO 2022 explored the potential impact of the type of surgical resection, baseline disease burden, and use of adjuvant chemotherapy on DFS in the overall population [20].
Pembrolizumab was shown to generally improve DFS compared with placebo regardless of the type of surgical resection (bilobectomy, lobectomy or pneumonectomy), degree of lymph node involvement (pN 0, 1, or 2), tumor size (≤ 4 vs. > 4 cm), and type and extent of adjuvant chemotherapy (carboplatin- or cisplatin-based; 1-2 or 3-4 cycles). Together with the overall efficacy and safety findings, these data support the benefit of adjuvant pembrolizumab for stage IB (tumor size ≥ 4 cm) to IIIA NSCLC following complete resection and, if recommended, adjuvant chemotherapy.
Unresectable stage III tumors: KEYNOTE-799
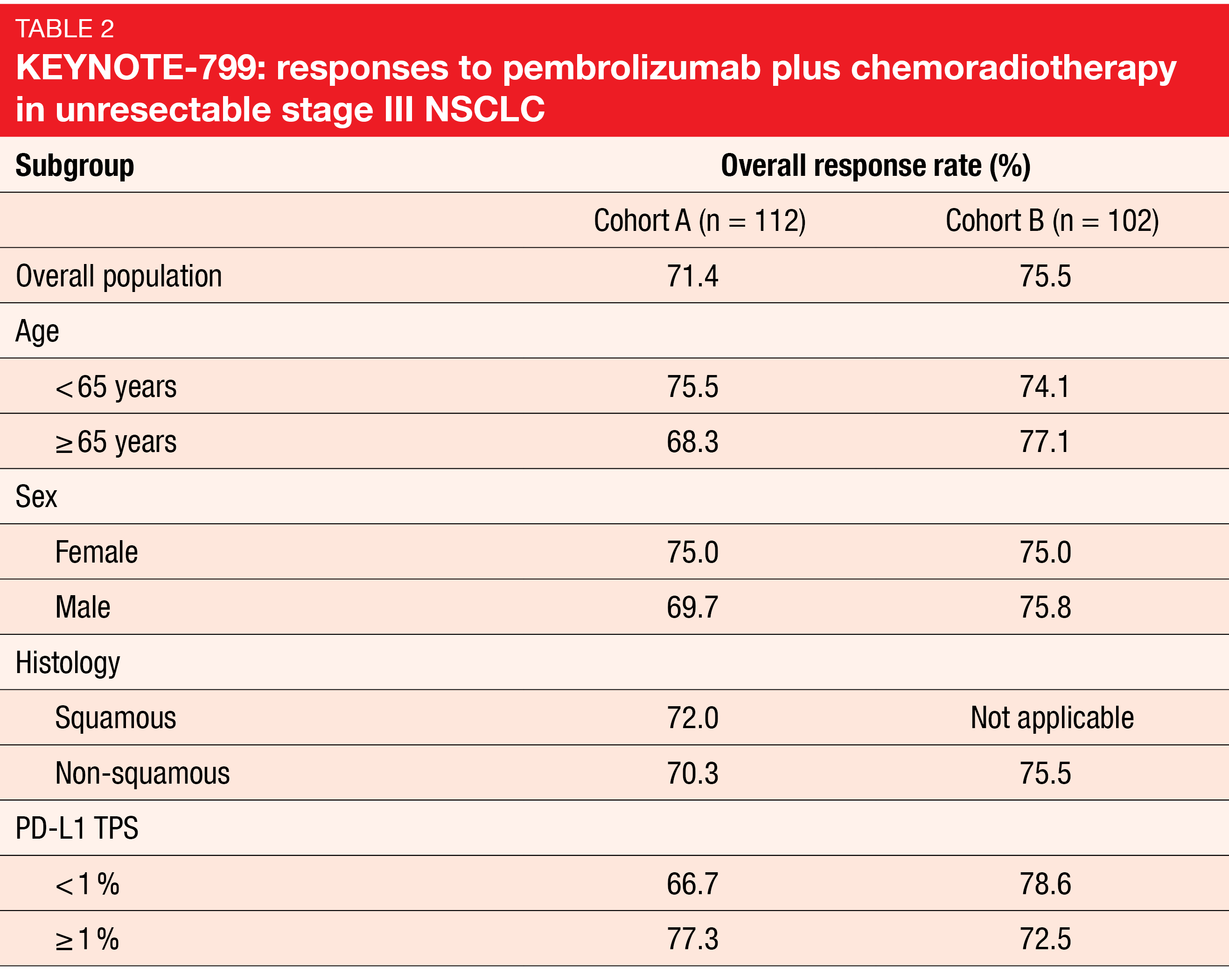
Outcomes for unresectable stage III NSCLC patients remain poor, and new strategies are required. The open-label, non-randomized, global, phase II KEYNOTE-799 study examined the combination of pembrolizumab with concurrent CRT for 3-4 cycles followed by pembrolizumab monotherapy for up to 17 cycles in patients with stage IIIA-C, unresectable, locally advanced, previously untreated NSCLC. Cohort A included 112 patients with squamous and non-squamous NSCLC, while Cohort B contained 102 individuals with non-squamous NSCLC only. The primary analysis of the trial has shown ORRs of 71 % in both cohorts and manageable safety [21]. At ASCO 2022, Reck et al. presented updated outcomes after 1 year of additional follow-up for all enrolled patients [22].
After more than 2 years of follow-up, the treatment continued to demonstrate robust and durable responses, with additional responses observed since the last analysis. ORRs were 71.4 % and 75.5 % in Cohorts A and B, respectively (Table 2). The patients responded regardless of their PD-L1 tumor proportion scores and tumor histology. Median duration of response had not been reached yet in either cohort, which also applied to OS. In Cohort A, 64.3 % of patients were alive at 24 months, and 64.0 % responded to treatment at that time; in Cohort B, 71.2 % lived, and the 24-month response rate was 68.7 %. Median PFS was 30.6 months in Cohort A and had not been reached yet in Cohort B. The 2-year PFS rates amounted to 55.3 % and 60.6 %, respectively. With respect to safety, no new signals emerged with longer follow-up. Grade ≥ 3 pneumonitis occurred in 8.0 % and 6.9 %, respectively. Discontinuation of any treatment component was due to immune-mediated AEs and infusion reactions in 18.8 % and 11.8 %, respectively.
In their summary, the authors noted that pembrolizumab plus concomitant CRT followed by pembrolizumab represents a promising strategy for patients with previously untreated, locally advanced, stage III NSCLC. The ongoing phase III KEYLYNK-012 and KEYVIBE-006 studies are further investigating pembrolizumab in addition to other compounds in unresectable, locally advanced, stage III disease.
Consolidation with nivo or nivo/ipi
The open-label, randomized, non-comparative phase II BTCRC LUN 16-081 study evaluated consolidation therapy with single-agent nivolumab 480 mg Q4W for up to 6 cycles (Arm 1; n = 54) or nivolumab 240 mg Q2W plus ipilimumab 1 mg/kg Q6W for up to 4 cycles (Arm 2; n = 51) following concurrent CRT in patients with unresectable stage IIIA or IIIB NSCLC [23]. The patients had achieved at least stable disease following chemoradiation. Improvement of the 18-month PFS rate compared to historical controls was defined as the primary endpoint.
At 18 months, PFS was obtained by 63.7 % and 67.6 % of patients in Arms 1 and 2, respectively, with median PFS amounting to 25.8 and 25.4 months, respectively. Compared to the historical controls of CRT alone (Arm A) and CRT followed by durvalumab (Arm B), the 18-month PFS rates were significantly improved. Median OS had not been reached for either arm yet. At 24 months, 77.7 % and 80.6 % of patients were alive. The authors noted that both arms demonstrated promising PFS and OS despite the limited 6-month duration of consolidation therapy. Compared to nivolumab monotherapy, nivolumab/ipilimumab gave rise to higher rates of pneumonitis (grade ≥ 2, 31.4 % vs. 22.2 %; grade 3, 17.6 % vs. 9.3 %), although no grade 4/5 events were observed. Any grade ≥ 3 treatment-related AEs occurred in 27.5 % vs. 18.5 %.
REFERENCES
- NSCLC Meta-analyses Collaborative Group, Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014; 383(9928): 1561-1571
- Hellmann MD et al., Pathologic response after neoadjuvant chemotherapy in resectable non-small cell lung cancers: proposal for the use of “major pathologic response” as a surrogate endpoint. Lancet Oncol 2014; 15(1): e42-e50
- Forde P et al., Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. New Engl J Med 2022; 386(21):1973-1985
- Provencio-Pulla M et al., Nivolumab + chemotherapy vs. CT as neoadjuvant treatment for resectable stage IIIA-B non-small cell lung cancer: NADIM II trial. J Clin Oncol 40, 2022 (suppl 16; abstr 8501)
- Waser NA et al., Pathologic response as early endpoint for survival following neoadjuvant therapy (NEO-AT) in resectable non-small cell lung cancer (rNSCLC): Systematic literature review and meta-analysis. Ann Oncol 2020; 31(suppl 4): S744-S753
- Mouillet G et al., Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non-small-cell lung cancer: combined analysis of two IFCT randomized trials. J Thorac Oncol 2012; 7(5): 841-849
- Provencio-Pulla M et al., Neoadjuvant nivolumab + platinum-doublet chemotherapy vs chemotherapy for resectable (IB-IIIA) non-small cell lung cancer: association of pathological regression with event-free survival in CheckMate 816. J Clin Oncol 40, 2022 (suppl 17; abstr LBA8511)
- Pless M et al., Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 2015; 386(9998): 1049-1056
- Strupp R et al., Neoadjuvant chemotherapy and radiotherapy followed by surgery in selected patients with stage IIIB non-small-cell lung cancer: a multicentre phase II trial. Lancet Oncol 2009; 10(8): 785-793
- Girard N et al., Is neoadjuvant chemoradiotherapy a feasible strategy for stage IIIA-N2 non-small cell lung cancer? Mature results of the randomized IFCT-0101 phase II trial. Lung Cancer 2010; 69(1): 86-93
- Katakami N et al., A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903). Cancer 2012; 118(24): 6126-6135
- Shah AA et al., Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg 2012; 93(6): 1807-1812
- Akhdar M et al., Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy for patients with stage III-N2M0 non-small cell lung cancer: a population-based study. J Clin Oncol 40, 2022 (suppl 16; abstr 8503)
- Provencio M et al., Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020; 21(11): 1413-1422
- Rothschild SI et al., SAKK 16/14: Durvalumab in Addition to Neoadjuvant Chemotherapy in Patients With Stage IIIA(N2) Non-Small-Cell Lung Cancer-A Multicenter Single-Arm Phase II Trial. J Clin Oncol 2021; 39(26): 2872-2880
- Shu CA et al., Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020; 21(6): 786-795
- Qiu Fuming et al., Two cycles versus three cycles of neoadjuvant sintilimab plus platinum-doublet chemotherapy in patients with resectable non-small cell lung cancer (neoSCORE): a randomized, single center, two-arm phase II trial. J Clin Oncol 40, 2022 (suppl 16; abstr 8500)
- Heiden BT et al., Intraoperative quality metrics and association with survival following lung cancer resection. J Clin Oncol 40, 2022 (suppl 16; abstr 8502)
- Paz-Ares L et al., Pembrolizumab versus placebo for early-stage non-small cell lung cancer following complete resection and adjuvant chemotherapy when indicated: randomized, triple-blind, phase 3 EORTC-1416-LCG/ETOP 8-15 – PEARLS/KEYNOTE-091 study. Ann Oncol 2022; 33(4): 451-453
- O’Brien MO et al., EORTC-1416-LCG/ETOP 8-15 – PEARLS/KEYNOTE-091 study of pembrolizumab versus placebo for completely resected early-stage non-small cell lung cancer: outcomes in subgroups related to surgery, disease burden, and adjuvant chemotherapy use. J Clin Oncol 40, 2022 (suppl 16; abstr 8512)
- Jabbour SK et al., Pembrolizumab plus concurrent chemoradiation therapy in patients with unresectable, locally advanced, stage III non–small cell lung cancer: the phase 2 KEYNOTE-799 nonrandomized trial. JAMA Oncol 2021; 7(9): 1-9
- Reck M et al., Two-year update from KEYNOTE-799: pembrolizumab plus concurrent chemoradiation therapy for unresectable, locally advanced, stage III NSCLC. J Clin Oncol 40, 2022 (suppl 16; abstr 8508)
- Durm G et al., Consolidation nivolumab plus ipilimumab or nivolumab alone following concurrent chemoradiation for patients with unresectable stage non-small cell lung cancer: BTCRC LUN 16-081. J Clin Oncol 40, 2022 (suppl 16; abstr 8509)
© 2022 Springer-Verlag GmbH, Impressum
More posts
Deeper insights into combinations of immune checkpoint inhibitors with other drug classes
Various regimens consisting of anti-PD-(L)1 antibodies with or without chemotherapy have been approved for the first-line treatment of patients with advanced NSCLC that does not harbor genomic alterations. The analysis reported at ASCO 2022 by Akinboro et al. used pooled data from 12 pivotal studies to compare overall survival (OS) obtained with chemoimmunotherapy (n = 455) vs. immunotherapy (n = 1,298) in patients with ALK- and EGFR-negative tumors that showed ≥ 50 % PD-L1 expression.
Targeting KRASG12C, METex14, EGFR & ALK: new ways to further improve patient outcomes
KRASG12C mutations are found in approximately 14 % of patients with adenocarcinoma of the lung. Adagrasib, a covalent inhibitor of KRASG12C, has been developed to show a long half-life of 23 hours, dose-dependent pharmacokinetics, and CNS penetration. At ASCO 2020, Spira et al. reported data from a registrational phase II cohort of 116 patients with unresectable or metastatic, KRASG12C-mutated NSCLC included in the multi-cohort, phase I/II KRYSTAL-1 study.
The clinical care pathways in early-stage lung cancer are changing
Many immunotherapies are being studied in the neoadjuvant or perioperative setting, but the only one that has been reported in a phase III trial as neoadjuvant treatment to date is nivolumab. There are three prospective trials that assessed nivolumab in the resectable setting. The NADIM I trial was a groundbreaking phase II study that looked at nivolumab plus chemotherapy followed by resection and demonstrated impressive results regarding pathologic endpoints and two-year survival.
Early-stage NSCLC: perioperative strategies and approaches in the unresectable
Neoadjuvant chemotherapy has been shown to significantly prolong overall survival (OS) in resectable non–small-cell lung cancer (NSCLC), although the absolute 5-year survival is improved by as little as 5 %. Similarly, pathological complete responses (pCR) are infrequently achieved; 15 trials investigating preoperative chemotherapy revealed an overall median rate of 4 %.
Preface ASCO Lung Cancer 2022
After 2 years of virtual meetings in the midst of the COVID-19 pandemic, the Annual Meeting of the American Society of Clinical Oncology (ASCO) returned to its live format, as oncology experts from around the world gathered again in Chicago, USA, and virtually from 3rd–7th June 2022, to discuss the most exciting updates in the field of lung cancer.