Extensive-stage small-cell lung cancer: successful and less successful combination strategies
In the first-line treatment of patients with extensive-stage small cell lung cancer (ES-SCLC), the IMpower133 and CASPIAN trials have established the anti-PD-L1 antibodies atezolizumab and durvalumab, respectively, as standard-of-care treatment in addition to platinum-etoposide [1-3]. However, PD-L1 inhibitors can only prolong overall survival by approximately 2 months and disease progression eventually develops in most cases, which still implies a significant unmet need for new therapies to improve long-term outcomes [1, 3, 4]. Moreover, the efficacy of anti-PD-1 antibodies in patients with SCLC remains unclear.
ASTRUM-005: serplulimab plus chemotherapy
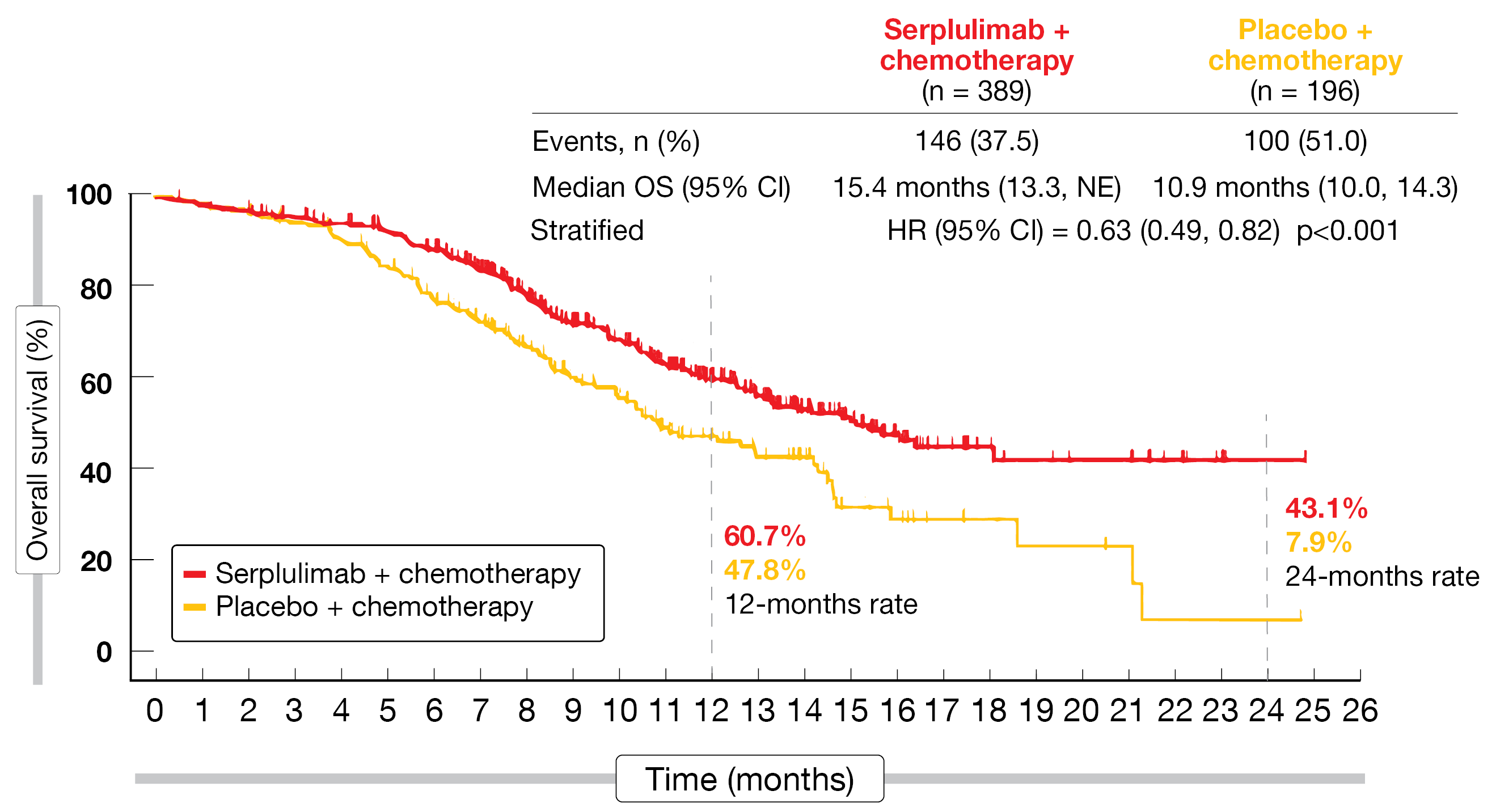
The novel anti-PD-1 antibody serplulimab has shown encouraging antitumor activity in patients with previously untreated unresectable or metastatic microsatellite instability-high or mismatch repair-deficient solid tumors [5]. Cheng et al. reported interim results from the randomized, double-blind, multicenter, phase III ASTRUM-005 study evaluating serplulimab 4.5 mg/kg plus carboplatin/etoposide Q3W for up to 4 cycles as first-line treatment of 389 patients with ES-SCLC [6]. After induction, the patients received serplulimab Q3W until disease progression. Meanwhile, the control arm (n = 196) was treated with placebo plus chemotherapy followed by placebo. Approximately 70 % of patients were Asian, and the majority showed no PD-L1 expression.
Serplulimab in addition to chemotherapy elicited consistent benefits across the efficacy endpoints, which included long-term effects. Regarding the primary outcome of overall survival (OS), the treatment gave rise to a 37 % reduction in mortality risk (15.4 vs. 10.9 months; HR, 0.63; p < 0.001; Figure). At 24 months, 43.1 % vs. 7.9 % of patients were alive. According to the subgroup OS analysis, all patient groups benefited from the addition of serplulimab. Similarly, the progression-free survival (PFS) results favored the combined approach, with median PFS of 5.7 vs. 4.3 months (HR, 0.48) and 12-month rates of 23.8 % vs. 6.0 %. Responses occurred in 80.2 % vs. 70.4 %; 3 patients (0.8 %) in the experimental arm achieved complete remission (vs. 0 % in the control arm). The median duration of response was longer with the serplulimab-based treatment (5.6 vs. 3.2 months; HR, 0.48).
The combination demonstrated a manageable safety profile that mainly included cytopenia, alopecia, nausea, and decreased appetite. Immune-related adverse events (AEs) were observed in 37.0 % (vs. 18.4 %), with the most common being hypothyroidism (11.6 %), hyperthyroidism (9.0 %), and rash (3.1 %). Treatment-related AEs (TRAEs) led to discontinuation in 4.9 % vs. 4.1 % and patient death in 0.8 % vs. 0.5 %. Grade ≥ 3 TRAEs occurred in 33.2 % vs. 27.6 %. No new safety signals were seen during the study.
Figure 1: Survival improvement with serplulimab plus chemotherapy versus placebo plus chemotherapy
SKYSCRAPER-02: no benefit with addition of tiragolumab
The randomized, double-blind, phase III SKYSCRAPER-02 trial tested the anti-TIGIT antibody tiragolumab in combination with atezolizumab and chemotherapy as first-line treatment in patients with ES-SCLC. TIGIT is an inhibitory immune checkpoint present on immune cells in many cancers and
is highly correlated with PD-1 expression [7]. Observations suggested that tiragolumab synergizes with other immunotherapies such as atezolizumab
to amplify the antitumor response [8, 9]. In SKYSCRAPER-02, the experimental treatment consisted of 4 cycles tiragolumab 600 mg plus atezolizumab 1,200 mg Q3W and carboplatin/etoposide, followed by maintenance treatment with tiragolumab plus atezolizumab until progression (n = 243). Patients in the control arm received placebo in addition to atezolizumab and carboplatin/etoposide, and the maintenance regimen contained placebo plus atezolizumab (n = 247). Most of the patients were Caucasian, while those of Asian origin made up approximately one quarter. OS and PFS in the primary analysis set (i.e., all randomized patients without presence or history of brain metastases at baseline) constituted the coprimary endpoint.
After a median follow-up of 14.3 months, the addition of tiragolimab was not shown to improve PFS (5.4 vs. 5.6 months for the experimental arm vs. the control arm; HR, 1.11, p = 0.3504) or OS (13.6 months in both arms; HR, 1.04; p = 0.7963) in the primary analysis set [10]. The same was true for the full analysis set, i.e., all randomized patients, regarding both PFS (5.1 vs. 5.4 months; HR, 1.08) and OS (13.1 vs. 12.9 months; HR, 1.02). The subgroup analysis of OS in the full analysis set did not identify any population that benefited from tiragolumab-based treatment. In the group of patients with brain metastases, median OS was 11.7 vs. 10.64 months (HR, 0.92). Likewise, no differences across the arms were noted for objective responses (70.8 % vs. 65.6 %) or median duration of response (4.2 vs. 5.1 months). Tiragolumab plus atezolizumab and chemotherapy was well tolerated, with the safety profile being similar to that of atezolizumab plus chemotherapy.
The authors concluded that based on these data, targeting TIGIT in the setting of ES-SCLC does not appear to be therapeutically relevant. The PFS and OS findings observed in the control arm support the results of the IMpower133 trial, thus further confirming this combination as a standard of care for the first-line treatment of patients with ES-SCLC. SKYSCRAPER-02 will continue to the planned primary OS analysis, and biomarker analyses are ongoing. Furthermore, tiragolumab is being investigated in NSCLC and other tumor types.
REFERENCES
- Horn L et al., First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018; 379: 2220-2229
- Liu SV et al., Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol 2021; 39(6): 619-630
- Paz-Ares L et al., Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomized, controlled, open-label, phase 3 trial. Lancet 2019; 394(10212): 1929-1939
- Rudin CM et al., Small-cell lung cancer. Nat Rev Dis Primers 2021; 7(1): 3
- Li J et al., Updated efficacy and safety results from the phase 2 study of serplulimab, a novel anti-PD-1 antibody, in patients with previously treated unresectable or metastatic microsatellite instability-high or mismatch repair-deficient solid tumors. J Clin Oncol 40, 2022 (suppl 16; abstr 2592)
- Cheng Y et al., ASTRUM-005: serplulimab, a novel anti-PD-1 antibody, plus chemotherapy versus chemotherapy as first-line treatment for extensive-stage small-cell lung cancer: an international randomized phase 3 study. J Clin Oncol 40, 2022 (suppl 16; abstr 8505)
- Johnston RJ et al., The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 2014; 26(6): 923-937
- Bendell JC et al., Phase Ia/Ib dose-escalation study of the anti-TIGIT antibody tiragolumab as a single agent and in combination with atezolizumab in patients with advanced solid tumors. Clin Cancer Res 2020; 80(suppl 16): abstract CT302
- Cho et al., Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol 2022; 23(6): 781-792
- Rudin CM et al., SKYSCRAPER-02: primary results of a phase III, randomized, double-blind, placebo-controlled study of atezolizumab + carboplatin + etoposide with or without tiragolumab in patients with untreated extensive-stage small cell lung cancer. J Clin Oncol 40, 2022 (suppl 17; abstr LBA8507)
© 2022 Springer-Verlag GmbH, Impressum