Targeting KRASG12C, METex14, EGFR & ALK: new ways to further improve patient outcomes
KRYSTAL-1: adagrasib in KRASG12C-mutated tumors
KRASG12C mutations are found in approximately 14 % of patients with adenocarcinoma of the lung [1]. Adagrasib, a covalent inhibitor of KRASG12C, has been developed to show a long half-life of 23 hours, dose-dependent pharmacokinetics, and CNS penetration [2, 3]. At ASCO 2020, Spira et al. reported data from a registrational phase II cohort of 116 patients with unresectable or metastatic, KRASG12C-mutated NSCLC included in the multi-cohort, phase I/II KRYSTAL-1 study [4]. These patients received adagrasib 600 mg BID after pretreatment with a PD-(L)1 inhibitor in combination or in sequence with chemotherapy. Their median number of prior lines was 2, with 22 % having received ≥ 3 lines. The overall response rate (ORR) constituted the primary endpoint.
Adagrasib demonstrated promising activity. The ORR amounted to 43 %, and 80 % of patients achieved disease control. Seventeen individuals were not evaluable due to having received post-baseline scans too early or study withdrawal prior to the first scheduled assessment; after elimination of their data, the ORR was 51 %. Responses were deep, with 75 % of responders achieving tumor reductions of > 50 %. The median time to response was 1.4 months, and responses lasted for a median of 8.5 months. At the time of data cutoff, treatment was ongoing in half of responders, with 33 % still responding. Median PFS and OS were 6.5 and 12.6 months, respectively. At 12 months, 51 % of patients lived, and 29 % were progression-free.
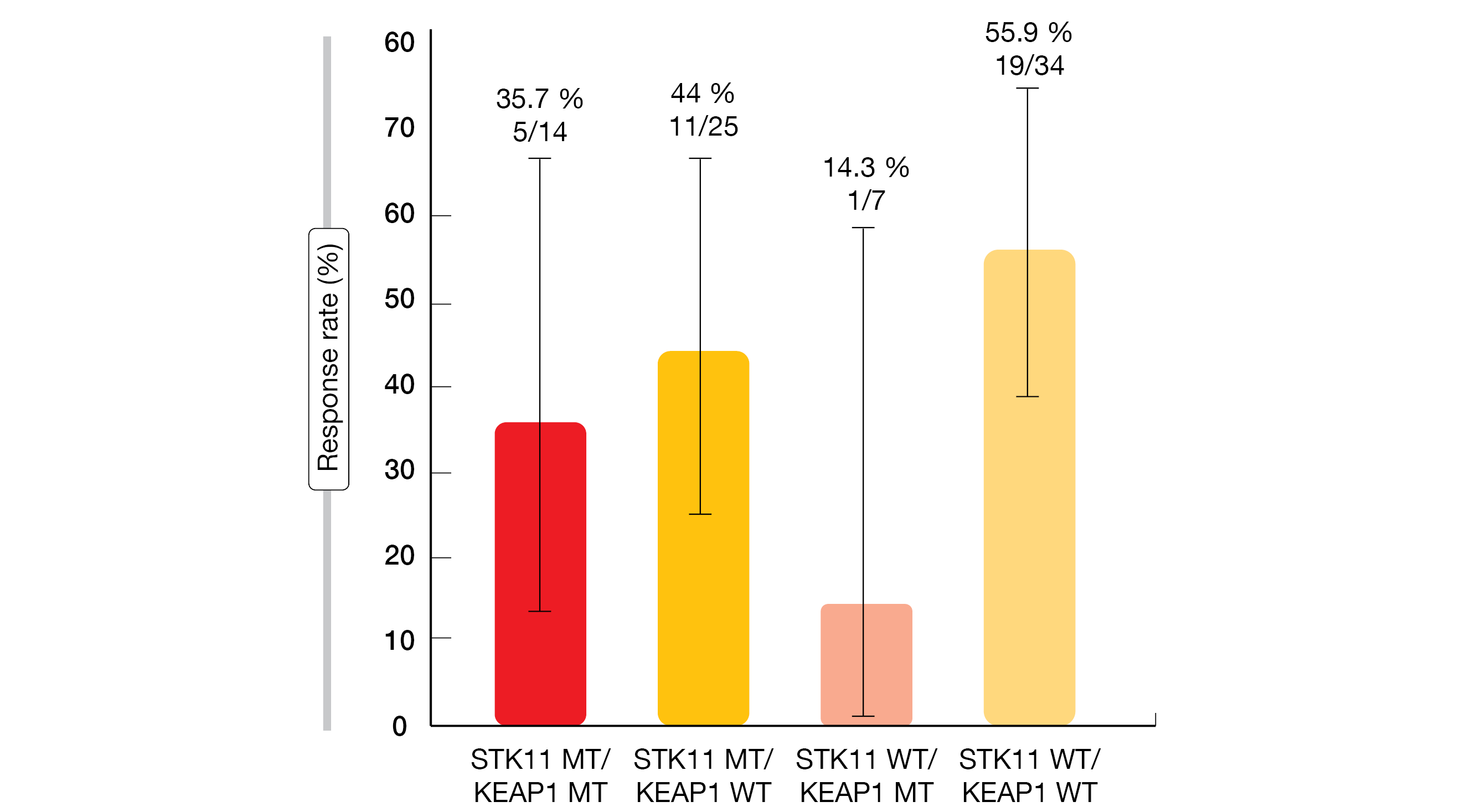
Pre-specified correlative analyses of co-mutations showed that response rates did not vary to a noticeable degree according to the presence of STK11, KEAP1, TP53, or CDKN2A mutations. Only patients who had STK11 wildtype plus KEAP1 mutation in addition to their KRASG12C mutation showed substantially reduced responses (Figure 1). The PD-L1 expression did not affect the outcomes.
Adagrasib demonstrated a manageable safety profile, with AEs being mainly grade 1 and 2. Treatment-related AEs (TRAEs) led to dose reductions and dose interruptions in 52 % and 61 %, respectively. Only 7 % of patients discontinued treatment due to TRAEs. Two grade 5 events occurred, which were cardiac failure and pulmonary hemorrhage.
Figure 1: KRYSTAL-1: effect of the STK11/KEAP1 mutation status on responses to adagrasib in pretreated patients with KRASG12C-mutant NSCLC
CNS activity of adagrasib
Brain metastases are common in the setting of KRAS-mutant NSCLC and are associated with poor prognosis [5]. Patients with adequately treated, stable CNS lesions were allowed to enroll in the phase II cohort of the KRYSTAL-1 trial. Among these, 19 and 13 had non-target lesions only and target lesions, respectively. Overall, the intracranial ORR amounted to 33 %; complete intracranial remission occurred in 15 %, partial remission in 18 %, and disease stabilization in 52 %, which added up to an intracranial disease control rate of 85 %. The patients showed a median intracranial duration of response of 11.2 months and a median intracranial PFS of 5.4 months. In the group with target lesions at baseline, the intracranial ORR was as high as 54 %.
The phase IB of the KRYSTAL-1 study included patients with active, untreated CNS metastases. According to the findings reported by Sabari et al., adagrasib showed encouraging and durable CNS-specific activity in a radiographically evaluable population of 19 individuals [6]. The intracranial ORR achieved in this group was 32 %, with a disease control rate of 84 %. Patients with non-target lesions only (n = 4) responded in 50 %, and those with both target lesions and non-target lesions (n = 15), in 27 %. Median intracranial duration of response had not been reached yet, and median intracranial PFS was 4.2 months. The researchers also assessed the concordance between systemic and intracranial disease control, which was 88 %.
Grade 1/2 TRAEs were observed in 60 % of patients. No grade 4/5 events occurred. Dose reduction/interruption and discontinuation resulted in 48 % and 4 %, respectively. CNS-specific TRAEs included dizziness (20 %) and grade 1/2 aphasia and insomnia (4 %). The authors stressed that adagrasib is the first KRASG12C inhibitor to demonstrate clinical activity in patients with KRASG12C-mutant NSCLC with treated and untreated CNS metastases. At present, the confirmatory phase III KRYSTAL-12 trial is evaluating adagrasib compared to docetaxel in previously treated patients with KRASG12C-mutated NSCLC (NCT04685135).
Amivantamab in METex14-positive disease
Approximately 3 % of NSCLCs harbor MET exon 14 (METex14) skipping mutations that lead to constitutive activation of the MET pathway [7, 8]. Amivantamab, a EGFR-MET bispecific antibody, is currently being evaluated in primary MET-driven tumors. The phase I CHRYSALIS study has established amivantamab 1,050 mg and 1,400 mg in patients with < 80 kg and ≥ 80 kg body weight, respectively, as the recommended phase II dose. In the dose expansion part of the trial, the safety and efficacy of amivantamab was tested in patients with METex14 skipping mutation. Among 55 individuals whose data were presented at ASCO 2022, 9 were treatment-naïve, while no prior MET inhibitor therapy had been administered in 18 cases and 28 patients had previously received MET inhibition [9]. In the MET-inhibitor–pretreated group, the median number of prior lines was 3 (range, 1-10), and 25 % had a history of brain metastases.
A total of 46 patients were efficacy-evaluable, demonstrating an ORR of 33 %. The treatment-naïve cohort showed the highest ORR of 57 %, followed by the patients who had received no prior MET inhibitor therapy (47 %). In the MET-inhibitor–pretreated group, only 17 % responded. The clinical benefit rates were 59 % overall and 71 %, 53 % and 58 % for the treatment-naïve, MET-naïve and MET-pretreated, respectively. Over time, amivantamab therapy gave rise to durable responses. Median duration of response had not been reached yet. Eleven of the 15 responders remained on treatment, and the patient with the longest response was still receiving amivantamab at 76 weeks. Median PFS was 6.7 months in the entire group. In the treatment-naïve cohort, median PFS had not been reached yet, and for the other two groups, it was 8.3 and 4.2 months.
The safety profile of amivantamab in the METex14-positive patient cohort was consistent with the larger CHRYSALIS safety population. Most AEs were grade 1 or 2, with a low discontinuation rate of 5 %. Pneumonitis/ILD emerged in 4 %. Rash-related events were observed in 76 % and were mainly low-grade. According to the authors, these preliminary results suggest that the monotherapy activity of amivantamab in patients with primary METex14-positive NSCLC is consistent with that of approved MET tyrosine kinase inhibitors (TKIs). The findings confirmed the independent, targeting action of each arm of the bispecific agent. Enrollment in the METex14-mutated cohort of the CHRYSALIS study is ongoing.
CHRYSALIS II: amivantamab plus lazertinib
The CHRYSALIS-2 study is exploring the combination of amivantamab with the highly selective, third-generation EGFR TKI lazertinib that is CNS-active and effective against both activating EGFR mutations and the resistance mutation T790M [10, 11]. Shu et al. presented the data after full enrollment of Cohort A, which comprised 162 patients with EGFR-mutant NSCLC who had progressed on osimertinib and platinum-based chemotherapy [12]. Their median number of prior therapy lines was 3 (range, 2-14). Twenty-eight percent had received ≥ 4 lines. Most had initially been treated with a first- or second-generation EGFR TKI followed by osimertinib and platinum-based chemotherapy (42 %). Brain metastases were present at baseline in 41 %.
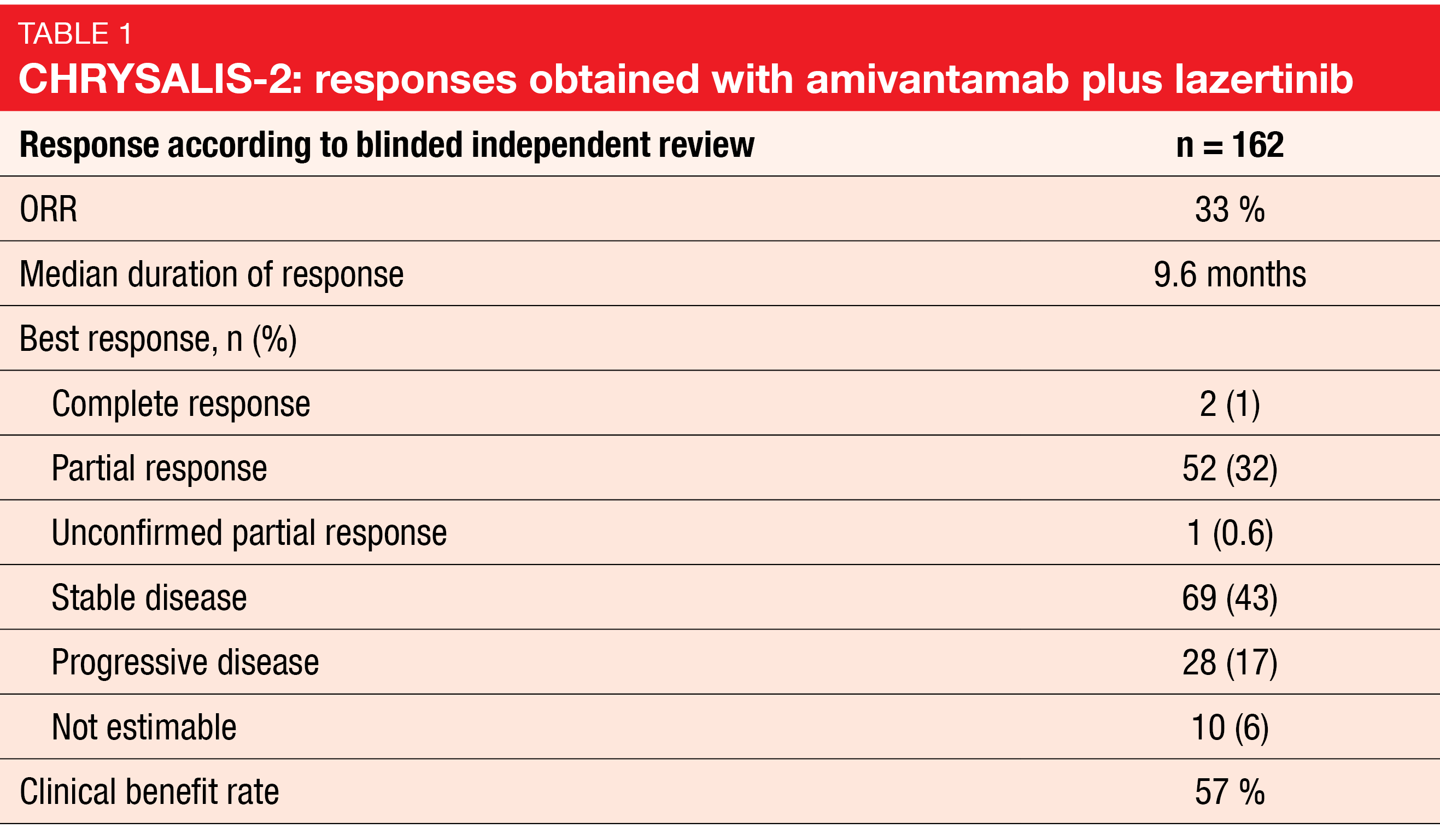
According to blinded independent review, 33 % of patients responded to the combined treatment, with responses lasting for a median of 9.6 months (Table). The clinical benefit rate was 57 %. When viewed by prior therapy, patients who had received osimertinib followed by chemotherapy showed a 21 % ORR, while those after the EGFR TKI/osimertinib/chemotherapy sequence responded in 36 %. Heavily pretreated patients and those treated out of sequence had an ORR of 39 %. At the time of clinical cutoff, 30 of 54 responders remained on treatment; in 27 of these, the response duration was ≥ 6 months. For 69 patients with stable disease as best response, 8 remained on treatment, and disease stabilization had been present for ≥ 6 months in 15 individuals. Median OS and PFS amounted to 14.8 and 5.1 months, respectively. A retrospective, exploratory CNS analysis among 27 patients with untreated baseline brain metastasis who had completed ≥ 1 post-baseline brain scan yielded complete clearance in 26 %. In the remaining 74 %, neither clearance nor progression occurred.
The safety profile of amivantamab plus lazertinib was consistent with prior reports. Pneumonitis/ILD was observed in 7 % of patients, with 4 % rated as grade ≥ 3, although no grade 5 events occurred. Eighty percent developed cumulative rash-related AEs (grade ≥ 3, 10 %). Most AEs were grade 1/2. AEs necessitated dose interruptions, reductions, and discontinuations of both amivantamab and lazertinib in 35 %, 9 %, and 7 %, respectively.
As the scientists noted, the combination evoked clinically significant and durable antitumor activity without biomarker selection in a population that had exhausted the standard of care and included heavily pretreated patients. The effects were comparable to those previously reported in a post-osimertinib, chemotherapy-naïve population [13]. This suggests that intervening chemotherapy does not impact the activity of the amivantamab/lazertinib regimen. The CHRYSALIS-2 study is ongoing, as well as the randomized phase III MARIPOSA trial (amivantamab plus lazertinib in the frontline setting) and the MARIPOSA-2 trial (amivantamab, lazertinib, carboplatin and pemetrexed after osimertinib).
Inhibition of EGFR ex20ins mutations with CLN-081
EGFR exon 20 insertion (ex20ins) mutations are found in approximately 2-3 % of all NSCLC cases [14] and are indicative of a poorer prognosis compared to tumors with more common EGFR mutations [15]. As the therapeutic window between wildtype EGFR und EGFR ex20ins is narrow, agents currently approved for the treatment of these patients carry significant toxicity. Safer and more effective novel therapies remain an unmet medical need.
CLN-081 is an irreversible, oral EGFR inhibitor with broad-spectrum activity against EGFR mutations that shows selectivity for the inhibition of EGFR ex20ins mutant vs. wildtype EGFR [16, 17]. A phase I/II, dose-escalation, dose-expansion study is currently assessing CLN-081 in patients with recurrent or metastatic, EGFR ex20ins-mutant NSCLC. Yu et al. reported the results for 73 patients who were enrolled across doses ranging from 30 to 150 mg BID [18]. This was a heavily pretreated population, with 66 % having received ≥ 2 prior lines of therapy. Previous EGFR TKI treatment had been administered in 36 %, including 3 patients (4 %) who had received the EGFR ex20ins-targeting agents poziotinib and/or mobocertinib.
Enrollment at CLN-081 150 mg BID was discontinued after 11 patients based on toxicity. At doses < 150 mg, the safety profile proved amenable for long-term treatment, with most AEs being grade 1/2 and an absence of grade ≥ 3 rash or diarrhea. Dose reductions and discontinuations were uncommon at doses < 150 mg BID. Treatment-emergent pneumonitis occurred in 4 patients, but these cases were asymptomatic or confounded by comorbid medical illness. The pharmacokinetic profile was consistent with the clinical safety profile at 100 mg vs. 150 mg BID dose levels. For 8 hours post dose, a sustained pharmacokinetic exposure over GI50 for ex20ins EGFR was shown, while for wildtype EGFR, the exposure time over GI50 was limited at doses < 150 mg BID. Across dose levels, 38.4 % of patients achieved confirmed partial response, with the highest rate of 41 % observed in the 100 mg BID cohort. Stable disease resulted in 56.4 % in this group, and the median duration of response was > 21 months. Median PFS ranged from 8 months for the ≤ 65 mg BID dose to 12 months for the 100 mg BID dose. Three patients entered the study with CNS target lesions at baseline. One of them achieved both an intracranial and systemic response at cycle 6 and remains in partial response at cycle 16, while another continues on treatment after 1 year with stable disease both intracranially and systemically. Enrollment in the phase IIB portion of the study is planned for the second half of 2022. Moreover, studies in patients with active CNS metastases and those who have relapsed after prior EGFR ex20ins-targeted therapies are planned.
ALTA-1L: greater quality of response with brigatinib
The ALK inhibitor brigatinib has been approved for the first-line treatment of patients with locally advanced or metastatic ALK-positive NSCLC based on the open-label, randomized, phase III ALTA-1L study that compared brigatinib with crizotinib in patients after ≤ 1 prior systemic treatment line. An exploratory analysis of the trial data assessed the association of the depth of target lesion response to brigatinib with the outcomes [19]. This showed that the proportion of patients with the highest target lesion shrinkage of 76 % to 100 % was considerably larger in the brigatinib arm, where it represented the majority of patients (56 %), than in the crizotinib arm (34 %). The difference between brigatinib and crizotinib was significant according to Cochran-Armitage trend analysis (p < 0.0001) and Chi-square analysis (p = 0.0005). Median time to the deepest target lesion regression in confirmed responders was 14.6 and 7.4 months with brigatinib and crizotinib, respectively.
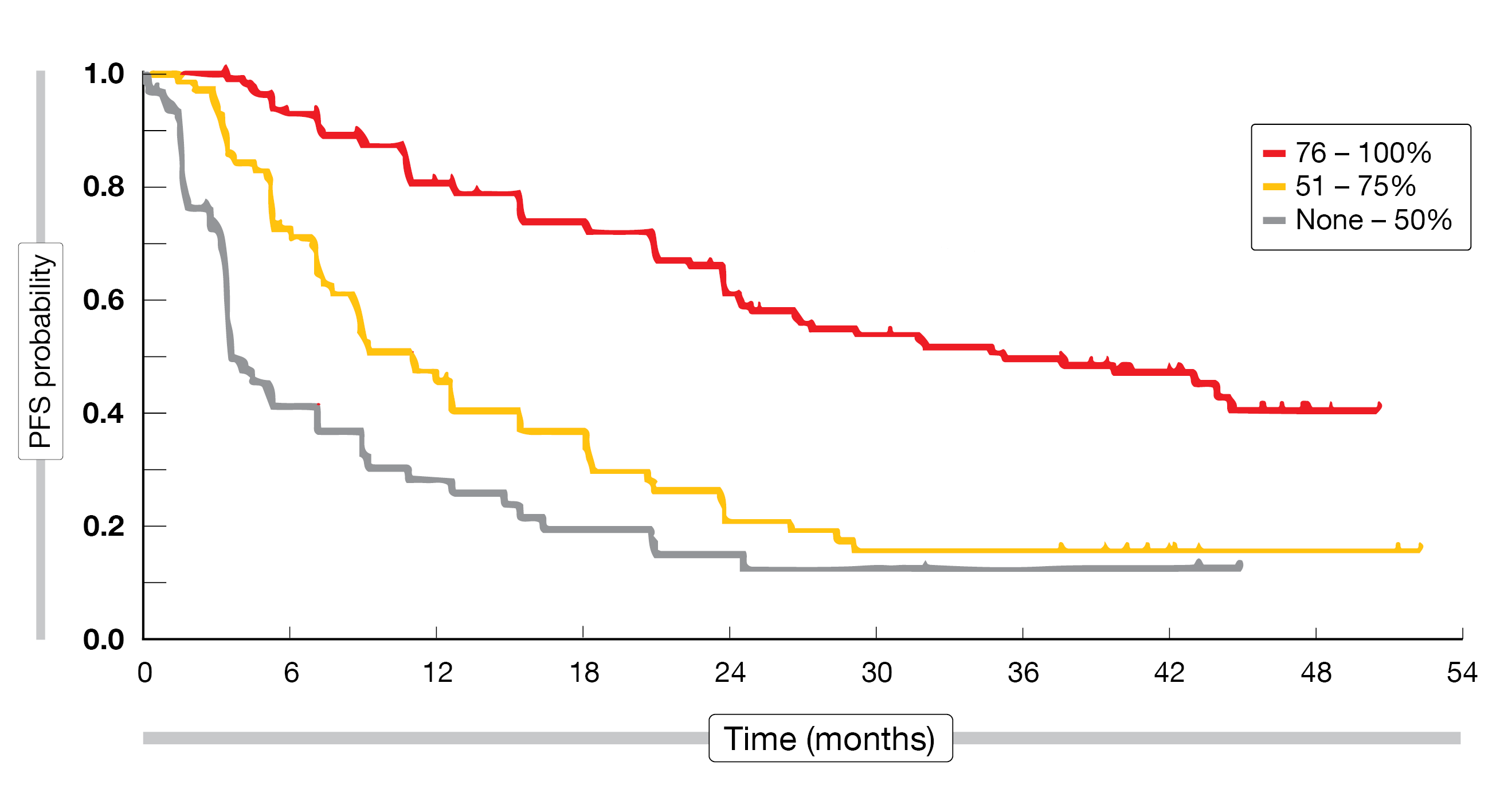
The researchers established a correlation between the depth of response and PFS. Across treatments, median PFS was shortest (i.e., 3.9 months) in the group with a maximum target lesion shrinkage of 50 % and longest (i.e., 35.5 months) in those with 76 % to 100 % shrinkage (Figure 2). In the group with 51 % to 75 % shrinkage, median PFS was 11.3 months. Patients with the highest degree of shrinkage had a 3-year PFS rate of 50 %, compared with 13 % and 16 % in the other groups. When viewed by treatment, the median PFS and 3-year PFS rates were numerically better in patients treated with brigatinib than in patients treated with crizotinib in the deepest response groups. Patients with > 75 % shrinkage had a significantly reduced risk of PFS or OS events compared to patients with ≤ 50 % target lesion shrinkage irrespective of treatment. The authors concluded that further evaluation of the relationship between depth of target lesion response and long-term PFS/OS and its potential as an early readout surrogate for prolonged benefit is warranted.
Figure 2: ALTA-L1: progression-free survival according to best target lesion shrinkage (pooled analysis for brigatinib and crizotinib)
Subsequent therapies after lorlatinib and crizotinib
The third-generation ALK TKI lorlatinib has demonstrated significant improvement in PFS over crizotinib in patients with previously untreated, ALK-positive, stage IIIB/IV NSCLC in the ongoing, international, randomized, phase III CROWN study [20, 21]. Median PFS had not been reached with lorlatinib and was 9.3 months with crizotinib (HR, 0.27) [21]. At ASCO 2022, data were reported on the efficacy of subsequent therapies following discontinuation of the ALK TKIs [22]. Sixty-one percent and 8.2 % of patients were still receiving lorlatinib and crizotinib, respectively, at data cutoff.
At least 1 subsequent anticancer treatment had been administered in 22.1 % vs. 70.1 %. ALK TKIs constituted the most commonly used first subsequent anticancer drugs in both arms (63.6 % vs. 93.2 %). Chemotherapy as first subsequent treatment was used in 36.3 % vs. 2.9 %. Median duration of treatment on the first subsequent systemic anticancer therapy was 9.6 vs. 13.3 months.
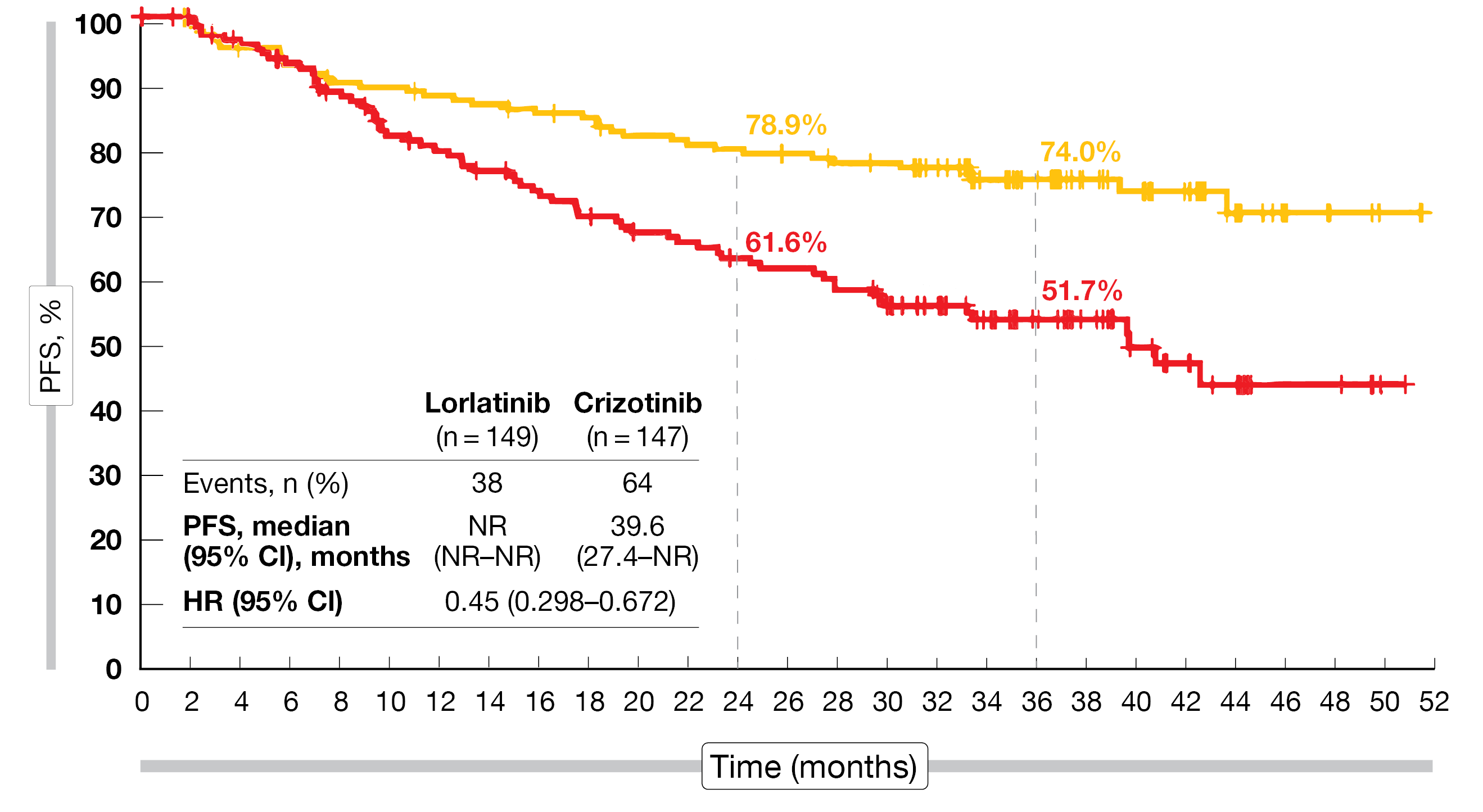
Subsequent systemic anticancer treatment offered clinical benefits in both arms, with response rates of 24.2 % vs. 15.5 %. Complete responses resulted in 6.1 % vs. 1.0 %, and partial responses in 18.2 % vs. 14.6 %. Moreover, the researchers assessed PFS2, which was defined as the time from randomization to disease progression on the first subsequent systemic anticancer therapy or death due to any cause. According to the PFS2 analysis, the clinical benefit was prolonged following lorlatinib vs. crizotinib and was maintained with subsequent systemic therapies. While median PFS2 had not been reached yet in the lorlatinib arm, it was 39.6 months in the crizotinib arm (HR, 0.45; Figure 3). Subsequent systemic therapy is ongoing in 30.3 % and 45.6 % of patients previously treated with lorlatinib and crizotinib, respectively.
Figure 3: Prolonged PFS2 with lorlatinib compared to crizotinib on subsequent systemic treatment
ALEK-B: alectinib plus bevacizumab
The combined administration of the ALK TKI alectinib 600 mg BID with the anti-VEGF antibody bevacizumab 15 mg/kg Q3W is being assessed in untreated patients with ALK-positive NSCLC in the open-label, phase II ALEK-B trial [23]. Between April 2020 and December 2021, 37 patients were enrolled. After a median follow-up of 19.9 months, median PFS had not been reached yet, and the 24-month event-free survival rate was 97.2 %. All patients were alive at that timepoint, and all had objective responses. In 3 cases (8.3 %), complete responses had occurred. The median tumor size reduction at week 6 was -52.1 %. Five patients had brain metastases at baseline. All of those with measurable disease (n = 4) responded intracranially, with 2 patients achieving complete remission. The CNS event-free rate at 12 months was 100 %. Median duration of systemic response and CNS response was 15.8 and 13.07 months, respectively.
The most commonly noted AEs included diarrhea (48.6 %), transaminase elevation (40.5 %), fatigue (37.8 %), anemia (35.1 %), and constipation (24.3 %). Grade 1/2 hypertension and proteinuria associated with bevacizumab treatment occurred in 21.6 % and 13.5 % of patients, respectively. Twelve patients (32.4 %) experienced grade ≥ 3 TRAEs, with the most frequent being ALT increases (18.9 %), AST increases (16.2 %), creatinine elevations (5.4 %), and diarrhea (5.4 %).
Overall, the combination of alectinib and bevacizumab was shown to be a safe and highly effective first-line regimen, conferring promising findings for patients with brain metastases. The authors concluded that longer follow-up will clarify the role of the combination with respect to deferment of resistance and prolongation of patient survival. These results warrant a larger confirmatory trial, ideally a randomized phase III study.
REFERENCES
- Nassar AH et al., Distribution of KRASG12C somatic mutations across race, sex, and cancer type. N Engl J Med 2021; 384(2): 185-187
- Hallin J et al., The KRASG12C inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov 2020; 10(1): 54-71
- Jänne PA et al., KRYSTAL-1: updated safety and efficacy data with adagrasib (MRTX849) in NSCLC with KRASG12C mutation from a phase 1/2 study. EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics 2020
- Spira AI et al., KRYSTAL-1: activity and safety of adagrasib (MRTX849) in patients with advanced/metastatic non-small cell lung cancer harboring a KRASG12C mutation. J Clin Oncol 40, 2022 (suppl 16; abstr 9002)
- Tomasini P et al., EGFR and KRAS mutations predict the incidence and outcome of brain metastases in non-small cell lung cancer. Int J Mol Sci 2016; 17(12): 2132
- Sabari JK et al., Activity of adagrasib in patients with KRASG12C-mutated NSCLC and active, untreated CNS metastases in the KRYSTAL-1 trial. J Clin Oncol 40, 2022 (suppl 17; abstr LBA9009)
- Frampton GM et al., Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015; 5(8): 850-859
- Ma PC et al., c-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res 2003; 63(19): 6272-6281
- Krebs MG et al., Amivantamab in NSCLC patients with MET exon 14 skipping mutation: updated results from the CHRYSALIS study. J Clin Oncol 40, 2022 (suppl 16; abstr 9008)
- Ahn MJ et al., Lazertinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: results from the dose escalation and dose expansion parts of a first-in-human, open-label, multicentre, phase 1-2 study. Lancet Oncol 2019; 20(12): 1681-1690
- Kim SW et al., Intracranial anti-tumor activity of lazertinib in patients with advanced NSCLC who progressed after prior EGFR TKI therapy: Data from a phase I/II study. J Clin Oncol 38: 2020 (suppl; abstr 9571)
- Shu CA et al., Amivantamab and lazertinib in patients with EGFR-mutant non-small cell lung cancer after progression on osimertinib and platinum-based chemotherapy: updated results from CHRYSALIS-2. J Clin Oncol 40, 2022 (suppl 16; abstr 9006)
- Bauml J et al., Amivantamab in combination with lazertinib for the treatment of osimertinib-relapsed, chemotherapy-naïve EGFR mutant non-small cell lung cancer and potential biomarkers for response. J Clin Oncol 39, 2021 (suppl 15; abstr 9006)
- Burnett H et al., Epidemiological and clinical burden of EGFR exon 20 insertion in advanced non-small cell lung cancer: A systematic literature review. PLoS One 2021; 16(3): e0247620
- Leal JL et al., EGFR exon 20 insertion mutations: clinicopathological characteristics and treatment outcomes in advanced non-small cell lung cancer. Clin Lung Cancer 2021; 22(6): e859-e869
- Hasako S et al., TAS6417, A novel EGFR inhibitor targeting exon 20 insertion mutations. Mol Cancer Ther 2018; 17(8):1648-1658
- Udagawa H et al., TAS6417/CLN-081 is a pan-mutation-selective EGFR tyrosine kinase inhibitor with a broad spectrum of preclinical activity against clinically relevant EGFR mutations. Mol Cancer Res 2019; 17(11): 2233-2243
- Yu H et al., Phase 1/2a study of CLN-081 in NSCLC patients with EGFR exon 20 insertion mutations. J Clin Oncol 40, 2022 (suppl 16; abstr 9007)
- Camidge DR et al., Association of depth of target lesion response to brigatinib with outcomes in patients with ALK inhibitor-naïve ALK+ NSCLC in ALTA-1L. J Clin Oncol 40, 2022 (suppl 16; abstr 9072)
- Shaw AT et al., First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med 2020; 383(21): 2018-2029
- Solomon BJ et al., Updated efficacy and safety from the phase 3 CROWN study of first-line lorlatinib vs crizotinib in advanced anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC). AACR 2022, abstract CT223
- Solomon BJ et al., Progression-free survival with subsequent anticancer therapies from a phase 3 trial of lorlatinib in treatment-naïve patients with ALK+ advanced non-small cell lung cancer. J Clin Oncol 40, 2022 (suppl 16; abstr 9069)
- Arrieta O et al., Phase II study of alectinib combined with bevacizumab as first-line treatment in advanced NSCLC with confirmed ALK fusion: ALEK-B trial. J Clin Oncol 40, 2022 (suppl 16; abstr 9074)
© 2022 Springer-Verlag GmbH, Impressum