Early insights for CPI combinations in solid tumors
Ezabenlimab combined with anti-VEGF/Ang2 inhibitor
Immunotherapy using anti-PD-1 immune checkpoint inhibitors (CPIs), which is a major therapeutic option in oncology, can potentially achieve synergistic effects once combined with targeted therapies [1]. Drugs targeting proangiogenic factors, such as VEGF and angiopoietin 2 (ANG2), can improve therapeutic responsiveness through their immunosuppressive activity in the tumor environment [1]. Combining antiangiogenic agents with CPIs may improve patient outcomes [2]. In an ongoing phase IB trial (NCT03468426), this therapeutic approach led to a manageable safety and preliminary anti-tumor activity [3].
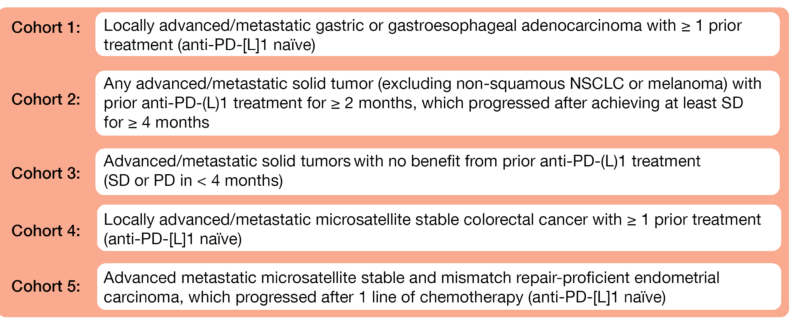
At the virtual ASCO 2021 meeting, Hussein et al. presented data from Module C of an ongoing open-label, phase II platform trial (NCT03697304) evaluating ezabenlimab – an anti-PD-1 antibody – in combination with BI 836880 – a humanized bispecific nanobody targeting VEGF and Ang2 – in previously treated advanced solid tumors [4]. The multicohort study already enrolled 150 patients into five patient cohorts (Figure 1). The patients received intravenously (IV) ezabenlimab (240 mg) and BI 836880 (720 mg) ever three weeks (Q3W). The objective response rate (ORR) per RECIST v1.1 is the primary study endpoint.
Among 60 treated patients as of April 2021, 62 % of them were male (median age, 62 years) and median duration of treatment was 70 days. Overall, 77 % of patients (n = 46) experienced any adverse events (AEs), most of them being mild or moderate; nausea (27 %), fatigue (23 %) and hypertension (20 %) were the most commonly reported AEs. Treatment-related adverse events (TRAEs) were observed in 47 % of patients, none of them being grade 4 or 5. Immune-related adverse events (irAEs) occurred in 7 % of patients and included rash and arthralgia (both of grade 1/G1), hypothyroidism (G2) and increased blood creatine phosphokinase (G3). Two patients had AEs that led to treatment discontinuation (G3 bile duct stone and G2 pain). Out of 33 patients with evaluable response, one had confirmed partial response (PR), 21 had stable disease and nine experienced progressive disease (PD).
The authors concluded that the combination of ezabenlimab with BI 836880 showed a manageable safety profile in this population. The study is currently recruiting in USA, Canada, and UK.
Figure 1: Cohort description of the phase II platform trial
Ociperlimab plus tislelizumab in advanced solid tumors
The dual targeting of ociperlimab – an anti-TIGIT inhibitor – and tislelizumab – an inhibitor of PD-1 –induced a synergistic immune cell activation and an enhanced antitumor activity in preclinical studies. This combination has been evaluated in a first-in-human phase I study AdvanTIG-105 in three Australian centers among 26 patients with advanced, metastatic unresectable solid tumors, for which standard therapy was ineffective, intolerable, or unavailable (NCT04047862). In this trial, the pharmacokinetics, the safety and the antitumoral activity of tislelizumab (200 mg, IV) combined with ociperlimab (IV, dose escalation between 50 and 900 mg, every three weeks) were investigated [5].
The median age of study participants was 56 years and 42 % were male. Two patients had a partial response (PR) (one with 900 mg ociperlimab, whose treatment is still ongoing and another with 450 mg, who unfortunately showed progression of disease after several months). The longest duration of stable disease was 54 weeks in one patient with 150 mg ociperlimab. A reduction in target lesions of more than 30 % was observed in three patients.
Overall, 96 % of patients (n = 25) experienced at least one treatment-emergent AE (TEAE) and 58 % of them (n = 15) had one immune-related TEAE or more. In the investigational group which received 900 mg of ociperlimab, three irAEs grade ≥ 3 occurred (colitis, decreased cortisol, and diabetic ketoacidosis). No dose-limiting toxicities (DLTs) were reported.
The authors concluded that this dual therapy showed a preliminary antitumoral activity and was well tolerated in patients with advanced solid tumors. The recommended phase II dose is therefore 900 mg ociperlimab IV combined with 200 mg tislelizumab IV three-weekly (Q3W).
Anti-LAG-3 monotherapy or combined with sintilimab
Lymphocyte-activation gene 3 (LAG-3) is a CPI involved in the response, activation and growth of T-cells [6]. The addition of anti-LAG-3 to an anti-PD-1 inhibitor might improve synergistically the antitumoral activity. This hypothesis was tested in a phase Ia/Ib dose-escalation study (NCT04085185) evaluating IBI110 (anti-LAG-3) and sintilimab (anti-PD-1) in patients with locally advanced, recurrent or metastatic solid tumors and presented at ASCO 2021 [7].
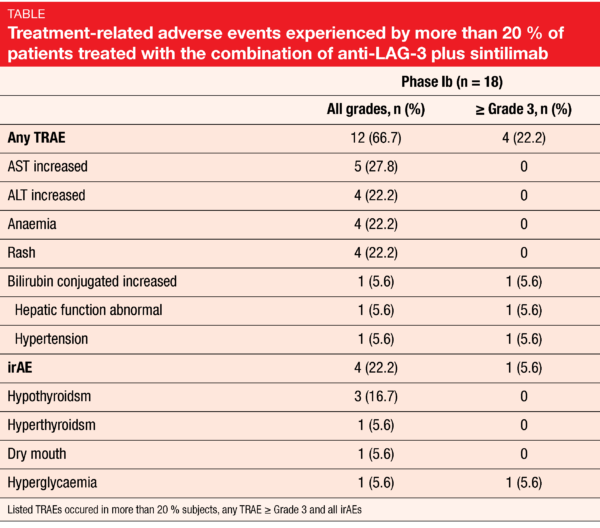
The dose-escalation of IBI110 alone was investigated in the phase Ia among 22 patients, while the phase Ib evaluated IBI110 plus sintilimab (200 mg, IV, Q3W) in 18 patients; a cross-over from IBI110 monotherapy to the combination group was allowed at disease progression. The study objectives were safety and tolerability, pharmacokinetics, pharmacodynamics, and antitumor activity of IBI110 according to RECIST v1.1. The median age was 61 years in both phases; both groups enrolled in majority male patients (63.6 vs 72.2 %, respectively) and the lung was the primary tumor location (54.5 vs 66.7 %, respectively). A PR was seen in three patients (1 patient with ovarian cancer in phase 1a, as well as 2 patients with small cell lung cancer and endometrial cancer in phase 1b). Five progressive patients under IBI110 monotherapy had a stable disease (SD) with the dual therapy after crossing over.
In the phase Ia, treatment-related adverse events (TRAEs) all grades were experienced by 40.9 % of patients, while grade ≥ 3 TREAs (anemia) occurred in 4.5 % of them. In the phase Ib with the combined therapy, a higher incidence of TRAEs any grade (66.7 %) and grade ≥ 3 (22.2 %); increased bilirubin conjugated, abnormal hepatic function and hypertension, 5.6 % each) were observed. Additionally, irAE (hyperglycemia) was experienced by one patient (5.6 %) (Table). No DLT was reported, and no adverse event led to discontinuation of the treatment in both groups.
IBI110 alone or combined with sintilimab showed a preliminary antitumor activity and an acceptable toxicity.
Eftilagimod alpha plus avelumab in advanced solid tumors
Eftilagimod alpha (efti) is a soluble LAG-3 and an MHC class II antagonist involved in the activation of the antigen-presenting cells after CD8 T-call activation. In combination with the anti-PD-1 CPI pembrolizumab, it showed already an encouraging antitumor activity by good tolerability in patients with metastatic melanoma [8]. Efti is currently under investigation in a phase II study in addition to paclitaxel to treat metastatic breast cancer [9].
In the stratum D of the INSIGHT investigator-initiated (IIT) platform trial (NCT03252938), the combination of efti plus avelumab – an anti-PD-L1 inhibitor – has been evaluated in patients with histologically confirmed locally advanced or metastatic solid tumors who did not receive more than three prior lines of therapy. The twelve enrolled patients received avelumab (800 mg, IV) plus efti (6 to 30 mg, SC) Q2W for a maximum of six months, followed by avelumab maintenance therapy Q2W for a maximum of a half year further [10].
With data-cut-off of January 22, 2021, a PR as best response was obtained in five patients, one SD with clinical progression, five disease progression according to RECIST v1.1 and one clinical progression. Moreover, the disease control rate (DCR) reached 50 %. Concerning the safety, among ten serious adverse events (SAEs) reported, none of them was related to the treatment. The most frequently observed grade ≥ 3 AEs were ileus (grade 3), nausea/vomiting (3), pain (3), hypokalemia (3), dysphagia (3), impaired hearing (4), sepsis (4), acute renal insufficiency (5), diffuse myocardial fibrosis (5) and urinary tract infection (3, related to avelumab).
As no unexpected AEs occurred, the researchers concluded that the combination of efti plus avelumab is feasible and safe.
Anti-PVRIG with or without nivolumab in advanced solid malignancies
COM701 is a novel first-in-class monoclonal antibody designed to block through a high affinity binding the interaction between the poliovirus receptor related immunoglobulin domain containing (PVRIG) and its ligand – PVRL2 [11]. The blockade of PVRIG induces an enhanced activation of T- and natural killer (NK) cells. At ASCO meeting 2021, Vaena et al. reported about results of an ongoing phase I study (NCT03667716) concerning the safety and tolerability, pharmacokinetics, and antitumor activity in patients with histologically confirmed locally advanced or metastatic solid tumors [12].
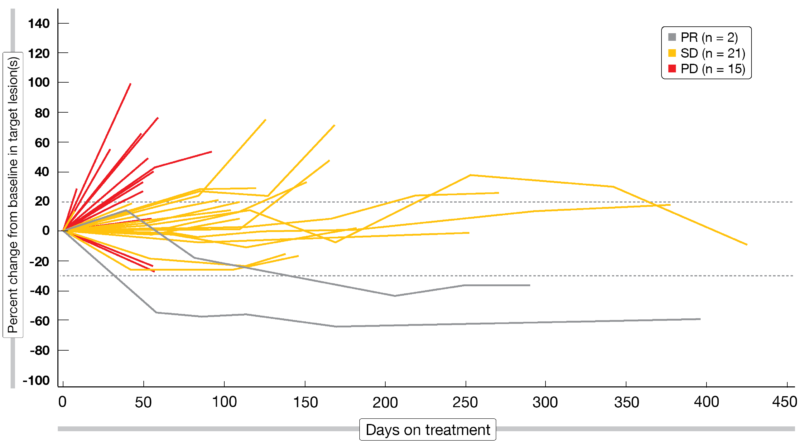
So far, 36 patients in monotherapy arm (A) received COM701 (0.01–20 mg/kg dose escalation, IV, Q3/Q4W). In arm B, 15 patients were administered additionally nivolumab (360 mg or 480 mg) to COM701 (0.3–20 mg/kg dose escalation) Q3/Q4W until the data-cut-off date (April 15, 2021). In the monotherapy group, the ORR was 3 % and the DCR 47 %, while in the combination group, these parameters amounted respectively to 13 % and 67 %. The Spider plot is showing the response in patients with a measurable disease (Figure 2). Overall, a CR, PR or SD was reached as best response in 52 % (n = 11/21) of patients with prior treatment refractory disease and in 72 % (n = 13/18) of those previously treated with a CPI.
At the maximal administered dose evaluated and chosen for the expansion cohorts (20 mg/kg COM701 alone or with 480 mg nivolumab), no DLTs were observed in both arms. Grade 3 TEAEs were experienced by 29 % (n = 11/38) of patients in arm A and in 44 % (n = 7/16) of those in the combination arm; they were concerning mostly ascites (8 %), dyspnea (5 %), nausea (3 %), diarrhea (3 %), vomiting (3 %) and abdominal pain (3 %) in arm A, as well as anemia (13 %), nausea (6 %) and back pain (6 %) in arm B. No TEAE grade 4 was reported, but one malignant neoplasm progression (breast cancer, TEAE grade 5) was observed in arm B.
The combination of COM701 plus nivolumab had an acceptable safety profile, was well tolerated, and showed a durable antitumor activity in extensively pretreated patients.
Figure 2: Spider plot: response among DLT-evaluable set (patients with measurable disease enrolled into dose escalation, patients in COM701 monotherapy expansion cohort).
Anti-TGF-β combined with spartalizumab in advanced solid tumors
The transforming growth factor beta (TGF-β) pathway signaling is involved in the immune regulation and plays especially a role in T-cell exhaustion, immune escape and resistance to immune checkpoint blockade [13]. Therefore, there is a scientific rationale to combine CPI with TGF-β inhibitor to improve the efficacy of the immunotherapy [14]. NIS793 is a novel anti-TGF-β monoclonal antibody and spartalizumab an anti-PD-1 inhibitor, whose safety has been already demonstrated in a first-in-human phase I study in patients with advanced solid tumors [15].
The results of a dose escalation and dose expansion first-in-man phase Ib (NCT02947165) of NIS793 first alone, than combined with spartalizumab by proven good safety have been recently presented at ASCO 2021 [16]. The safety and tolerability, as well as the determination of the recommended dose for expansion. Patients received initially NIS793 (0.3 to 1 mg/kg, Q3W) monotherapy; dose escalation continued then with NIS793 (0.3 to 30 mg/kg, Q3W) plus spartalizumab (300 mg, Q3W) or NIS793 (20 to 30 mg/kg, Q2W) plus spartalizumab (400 mg, Q4W).
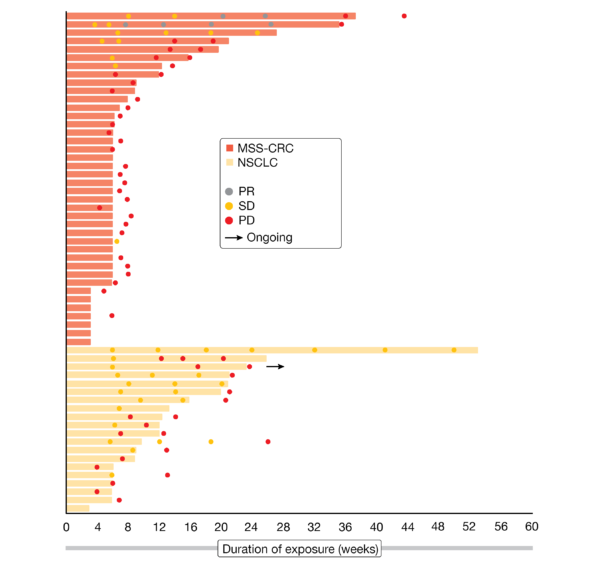
In total, 60 patients were treated in the dose escalation phase and 60 more (11 in monotherapy arm and 49 in the combination arm) in the dose expansion phase with the recommended dose (NIS793 2100 mg + spartalizumab 300 mg, Q3W) at the time of the analysis (December 1, 2020). The population was heavily pretreated, as nearly half of them had at least four prior therapies. Best response obtained were four PRs (3 %) and 28 SDs (23 %) – including twelve which lasted for more than four months – and 71 PDs (59 %). The duration of response (DoR) attained by MSS-CRC (microsatellite stable colorectal cancer) cases (40.8 % of all patients) and NSCLC (non-small cell lung cancer) cases (18.3 %) is shown in Figure 3. Moreover, gene expression and protein analyses in tumoral tissues suggested a modulation of the TGF- β pathway.
No DLTs were observed. Most of toxicities reported were grade 1 or 2, with dermatological ones being the most common (rash and pruritus); 13 patients (11 %) experienced grade 3 TRAEs, including rash (3 %), hyponatremia (2 %), as well as elevated lipase or amylase, adrenal insufficiency, and diarrhea (1 % each). No TRAEs grade 4 or 5 were observed. Treatment-related serious adverse events were observed in 7 % of patients.
The authors concluded that a preliminary antimoral activity was shown with this dual therapy, which was well tolerated in the recommended dose in patients with advanced malignant entities.
Figure 3: Duration of treatment in the expansion cohort.
CPI combinations in unselected cold tumors
Cold tumors are usually not responding to immunotherapy, as they are surrounded by cells able to suppress the immune response and keep T cells at distance. Therefore ORRs < 10 % were obtained in clinical trials with combined CPIs in this kind of tumors [17]. At ASCO 2021, an analysis performed in CPI-naïve patients with cold tumors treated between 2015 and 2021 with immune combinations was presented [18]. Clinicopathological data and antitumor activity were extracted from a prospective database.
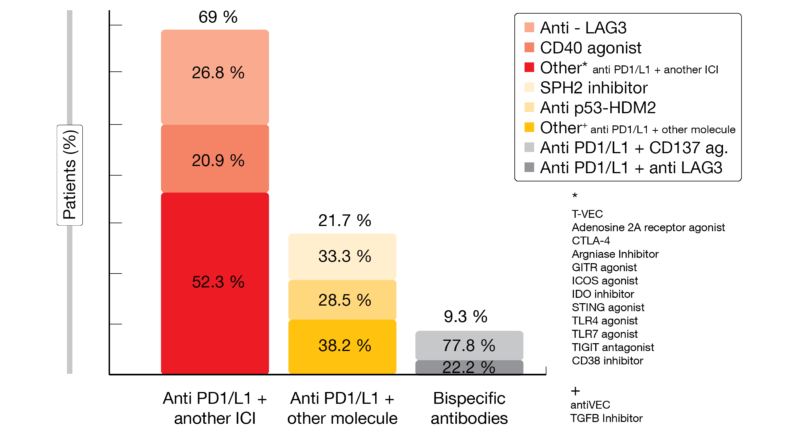
Among the 97 patients analyzed, median age was 62 years; the most represented tumor types were microsatellite stable (MSS) colorectal cancer (61 %) or ovarian cancer (14 %). In total, 69 % of patients received as combined treatment an anti-PD-1/L1 plus another CPI (most frequently anti-LAG3 and CD40 antagonist) (Figure 4). No patient achieved a response; the clinical benefit rate – defined as complete response (CR) + PR + SD for at least 4 months – was 15.3 %, with 58 patients (60 %) reaching a PD. Additionally, 20 patients (21 %) had a hyperprogressive disease (HPD) per RECIST v1.1 as previously defined by Matos et al. [19]. The median progression-free survival (PFS) was 1.9 months (95 % CI, 1.7-2.0) for the overall population and 5.9 months (95 % CI, 5.4-NR) for the CBR group. The median overall survival (OS) for the overall population was 7.6 months (range, 5.9-9.5), with a benefit for patients presenting a good LIPI (lung immune prognostic index) score (12.6 vs. 6.2 months; HR, 1.9; 95 % CI, 1.1-3.3; p = 0.02).
Out of 33 patients (34 %) who experienced immune-mediated toxicities, four patients (12 %) had grade 3 irAE (dry mouth, hypertransaminasemia, myocarditis and decreased neutrophil count) and 1 patient (3 %) grade 4 irAE (hyperglycemia). Overall, 33 % of patients who had PD developed irAEs.
The researchers concluded that CPI combinations showed only very limited activity in patients with unselected cold tumors and are associated with substantial risk for irAE and HPD.
Figure 4: Anti PD-1/PD-L1 combinations.
Zanidatamab plus chemotherapy, with or without tislelizumab
Patients with HER2 tumors tend to develop resistance and/or relapse towards HER2-targeted therapies; therefore, those patients are characterized by a poor survival and a lack of therapeutic responses [20, 21]. Zanidatamab is a novel anti-HER2 bispecific antibody that lead to enhanced tumor cell binding [22]; in a previously published early phase trial, Zanidatamab was well tolerated and showed antitumor activity in advanced HER2-positive tumors [23, 24]. The combination of HER2-targeting agents with chemotherapy led to improved survival [25]. Tislelizumab, which has been designed to overcome resistance to CPI, demonstrated good tolerability and antitumoral activity as monotherapy or combined with chemotherapy in advanced solid malignancies [26, 27].
At the ASCO Annual Meeting 2021, the design of a currently ongoing phase Ib/II trial was presented (NCT02892123) [28]. In cohort 1, zanidatamab (30mg/kg or 1800 mg, IV, Q3W) plus docetaxel (75 mg2, IV, Q3W) is evaluated as first-line therapy of patients with HER2+ metastatic breast cancer; in cohort 2, zanidatamab plus chemotherapy (Q3W) is combined to tislelizumab (200 mg, IV) in treatment-naïve patients with HER2+ advanced gastric/gastroesophageal junction adenocarcinoma (GC/GEJC). The co-primary study endpoints are safety and ORR per RECIST v1.1, while secondary endpoints include DoR, time to response, PFS, DCR, and OS. The study is intended to be conducted in twelve centers in Asia and to enroll approximately 50 patients.
REFERENCES
- Fukumura D et al., Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018; 15(5): 325-340.
- Allen E et al., Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med 2017; 9(385).
- Girard N et al., Phase Ib study of BI 836880, a VEGF/Ang2-blocking nanobody, in combination with BI 754091, an anti-PD-1 antibody: Initial results in patients (pts) with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2020; 38(suppl 15; abstr 9566).
- Hussein M et al., Platform trial of ezabenlimab (BI 754091), an anti-PD-1 antibody, in patients (pts)with previously treated advanced solid tumors: Combination with BI 836880, aVEGF/Ang2-blocking nanobody. J Clin Oncol 2021; 39(suppl 15; Abstr 2582).
- Frentzas S et al., AdvanTIG-105: Phase 1 dose-escalation study of anti-TIGIT monoclonal antibodyociperlimab (BGB-A1217) in combination with tislelizumab in patients withadvanced solid tumors. J Clin Oncol 2021; 39(suppl 15; abstr 2583).
- Lythgoe MP et al., Gene of the month: lymphocyte-activation gene 3 (LAG-3). J Clin Pathol 2021; epub ahead a print.
- Zhou C et al., Phase Ia/Ib dose-escalation study of IBI110 (anti-LAG-3 mAb) as a single agentand in combination with sintilimab (anti-PD-1 mAb) in patients (pts) withadvanced solid tumors. J Clin Oncol 2021; 39(suppl 15; abstr 2589).
- Atkinson V et al., Eftilagimod alpha, a soluble lymphocyte activation gene-3 (LAG-3) protein plus pembrolizumab in patients with metastatic melanoma. J Immunother Cancer 2020; 8(2).
- Dirix L et al., AIPAC: a Phase IIb study of eftilagimod alpha (IMP321 or LAG-3Ig) added to weekly paclitaxel in patients with metastatic breast cancer. Future Oncol 2019; 15(17): 1963-1973.
- Goetze T et al., Phase I INSIGHT platform trial: Advanced safety and effcacy data from stratumD evaluating feasibility and safety of eftilagimod alpha (soluble LAG-3 protein)combined with avelumab in advanced solid tumors. J Clin Oncol 2021; 39(suppl 15; abstr 2518).
- Li Y et al., Blockade of checkpoint receptor PVRIG unleashes anti-tumor immunity of NK cells in murine and human solid tumors. J Hematol Oncol 2021; 14(1): 100.
- Vaena DA. et al., COM701 with or without nivolumab: Results of an ongoing phase 1 study of safety, tolerability and preliminary antitumor activity in patients with advancedsolid malignancies. J Clin Oncol 2021; 39(suppl 15; abstr 2504).
- Bai X et al., Blocking TGF-β Signaling To Enhance The Efficacy Of Immune Checkpoint Inhibitor. Onco Targets Ther 2019; 12: 9527-9538.
- Grauel AL et al., TGFβ-blockade uncovers stromal plasticity in tumors by revealing the existence of a subset of interferon-licensed fibroblasts. Nat Commun 2020; 11(1): 6315.
- Naing A et al., A first-in-human phase 1 dose escalation study of spartalizumab (PDR001), an anti-PD-1 antibody, in patients with advanced solid tumors. J Immunother Cancer 2020; 8(1).
- Bauer T et al., Phase Ib study of the anti-TGF-β monoclonal antibody (mAb) NIS793 combinedwith spartalizumab (PDR001), a PD-1 inhibitor, in patients (pts) with advancedsolid tumors. J Clin Oncol 2021; 39(suppl 15; abstr 2509).
- Galon J et al., Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov 2019; 18(3): 197-218.
- Saavedra O et al., Evaluating the role of immune-checkpoint inhibitor (ICI) combinations in patients (pts) with unselected “cold” tumors enrolled in early clinical trials (CT). J Clin Oncol 2021; 39(suppl 15; abstr 2597).
- Matos I et al., Capturing Hyperprogressive Disease with Immune-Checkpoint Inhibitors Using RECIST 1.1 Criteria. Clin Cancer Res 2020; 26(8): 1846-1855.
- Wang J et al., Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct Target Ther 2019; 4: 34.
- Ayoub NM et al., Immunotherapy for HER2-positive breast cancer: recent advances and combination therapeutic approaches. Breast Cancer (Dove Med Press) 2019; 11: 53-69.
- Weisser N et al., The Bispecific Antibody Zanidatamab (ZW25) Has Unique Mechanisms of Action and Durable Anti-Tumor Activity in HER2-Expressing Cancers. AACR 2021 (Abstr 1005) 2021.
- Oh D et al., Safety, anti-tumour activity, and biomarker results of the HER2-targeted bispecific antibody ZW25 in HER2-expressing solid tumors. Ann Oncol 2019; 30 (suppl_9): ix22-ix29.
- Meric-Berstam F et al., Zanidatamab (ZW25) in HER2-positive biliary tract cancers (BTCs): Results from a phase I study. J Clin oncol 2019; 39(suppl 3; abstr 299).
- Pernas S et al., HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Ther Adv Med Oncol 2019; 11: 1758835919833519.
- Lee A et al., Tislelizumab: First Approval. Drugs 2020; 80(6): 617-624.
- Zhang T et al., The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother 2018; 67(7): 1079-1090.
- Lee K et al., Zanidatamab, an anti-HER2 bispecific antibody, plus chemotherapy with/withouttislelizumab as first-line treatment for patients with advanced HER2-positivebreast cancer or gastric/gastroesophageal junction adenocarcinoma: A phase1B/2 trial-in-progress. J Clin Oncol 2021; 39(suppl 15; abstr TPS2656).
© 2021 Springer-Verlag GmbH, Impressum