Novel approaches in gastric cancer
LEAP-005: lenvatinib plus pembrolizumab in gastric cancer
With more than 1 million newly diagnosed cases in 2020, gastric cancer was at the fifth place (5.6 %) of the most frequent malignant diseases and accounted for nearly 8 % of cancer deaths worldwide [1].
The multicohort, non-randomized, open-label, phase II LEAP-005 study (NCT03797326) was designed to evaluate the safety and efficacy of a combination – the anti-angiogenic multikinase inhibitor lenvatinib plus the anti-PD-1 antibody pembrolizumab – in patients with previously treated advanced solid tumors. Among the seven different cohorts, the results of the gastric cohort have been presented at the virtual scientific ASCO 2021 meeting [2]. The eligibility criteria were adults with confirmed metastatic and/or unresectable gastric cancer (GC), who received at least two prior lines of therapy, had measurable disease per RECIST v1.1, had a good performance status (ECOG PS 0‒1) and provided a tissue sample evaluable for PD-L1 (programmed cell death-ligand 1) expression. For up to 35 cycles (approximately 2 years) or until confirmed disease progression, unacceptable toxicity, or withdrawal of consent, lenvatinib was administered daily (20 mg, orally), while patients received pembrolizumab (200 mg, IV) every three weeks; if patients experienced a clinical benefit, lenvatinib could be continued beyond two years. Objective response rate (ORR) according to RECIST v1.1 criteria assessed by a blinded independent central review (BICR) and safety were the co-primary endpoints. Secondary endpoints included disease control rate (DCR), duration of response (DOR), PFS (progression-free survival), and OS (overall survival).
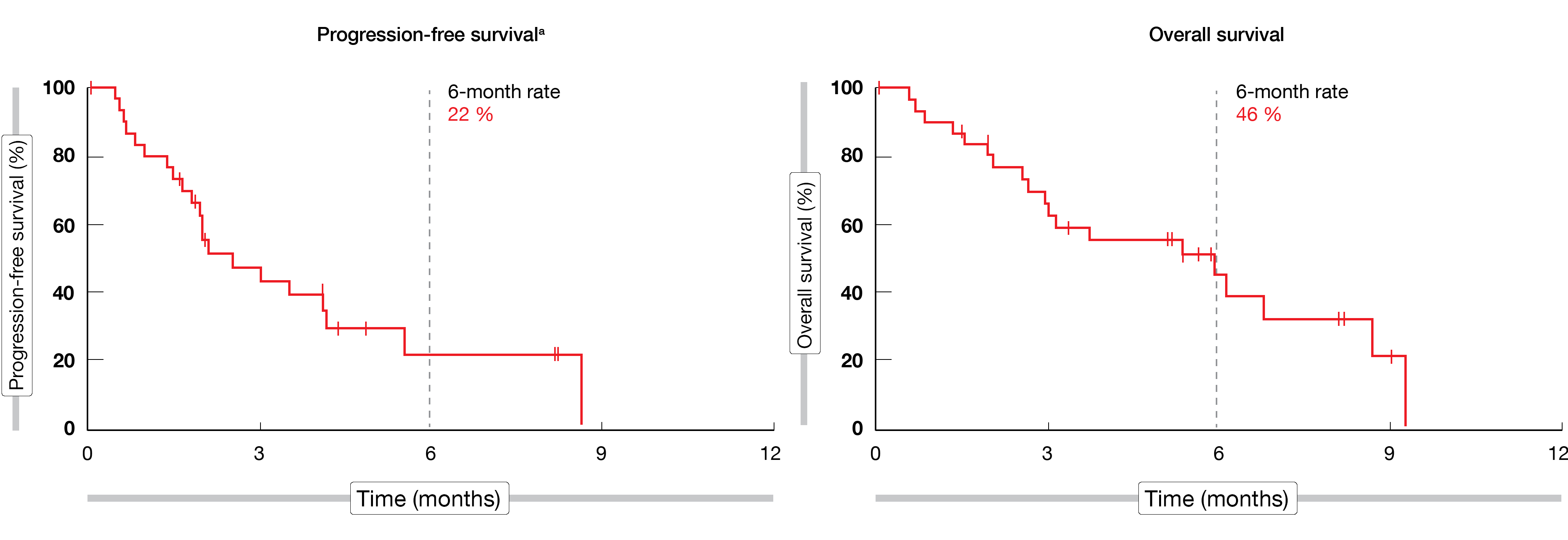
Among the 31 eligible patients recruited, the mean age was 62 years (range, 28-83), most of them (87 %) were male and 71 % had a combined PD-L1 positive score (CPS) ≥ 1. At the time of the analysis (April 10, 2020), patients were for 7.0 months (range, 1.9-11.9) on treatment. An ORR of 10 % (95 % CI, 2-26) and a DCR of 48 % (95 % CI, 30-67) were achieved by the study population; in total, one patient (3 %) experienced a complete response (CR), while two patients (6 %) experienced a partial response (PR) and twelve patients (39 %) a stable disease. Median DOR was not reached yet. A median PFS of 2.5 months (range, 1.8-4.2; 6-month rate, 22 %) and a median OS of 5.9 months (range, 2.6-8.7; 6-month rate, 46 %) were attained (Figure 1). The most common treatment-related adverse events (TRAEs) observed in ≥ 20 % of patients were diarrhea (26 %) and fatigue (26 %). Immune-mediated AEs, which were experienced by seven patients (22 %), included hypothyroidism (n = 5) and hyperthyroidism (n = 2). In total, twelve patients (39 %) experienced grade 3 AEs, while no AE of grade 4 severity was seen; one patient died due to a gastrointestinal hemorrhage considered to be tumor-related by the investigator.
According to these data, the combination of lenvatinib plus pembrolizumab demonstrated promising antitumor activity and a manageable safety profile; therefore, the enrollment in the gastric cohort has been extended to 100 patients.
Figure 1: Median PFS and median OS in the LEAP-005 study in patients with gastric cancer
PARALLEL 303: pamiparib as maintenance monotherapy in GC
Some gastric cancer present platinum sensitivity, as well as genomic instability (HRD, homologous recombination deficiency). Considering that cells with HRD are sensitive to poly (ADP-ribose) polymerase protein (PARP) inhibition [3], the use of PARP inhibitors as maintenance following platinum-based chemotherapy might be an efficient therapeutic strategy. The efficacy of PARP inhibitors in other cancer showing platinum sensitivity and higher levels of HRD has been already described, inclusive as maintenance therapy [4-6]. Pamiparib is an investigational PARP inhibitor showing sensitivity to HRD cells, which demonstrated its efficacy and tolerability in early-phase clinical study in advanced solid tumors [7, 8].
The double-blind, randomized, global, phase II PARALLEL 303 study (NCT03427814) compared the efficacy, safety, and tolerability of pamiparib against placebo as maintenance treatment in responders, who were defined as having a PR for ≥ 4 weeks or a CR after platinum-based fist-line chemotherapy [9]. Eligible patients had histologically confirmed inoperable locally advanced or metastatic GC (adenocarcinoma of the stomach or gastroesophageal junction) and were enrolled in 128 sites worldwide. The primary endpoint was PFS per RECIST v1.1, while time to subsequent treatment, ORR, DOR, time to response, OS and safety were the secondary endpoints. Data about PFS and safety were presented at ASCO 2021.
Overall, 136 patients were randomized 1:1 to receive either pamiparib (60 mg orally, twice daily) or placebo (twice daily) in 28-day cycles. At the time of the analysis, 51.5 % of patients in pamiparib were still on treatment against 16.9 % in the control arm. After a median follow-up of approximately 8.0 months in both arms, median PFS was longer with the investigational drug than with placebo, also not significantly different (3.7 vs 2.1 months; HR, 0.799; p = 0.1428). Likely, no significant clinical benefit was observed for median OS (10.2 with pamiparib vs 12.0 months with placebo) or ORR (7.7 vs 6.3, respectively) between both arms. Anemia (36.6 %) and nausea (32.4 %) among pamiparib-treated patients, as well as abdominal pain (18.5 %) in the control arm were the most frequently observed treatment-emergent adverse events (TEAEs). TEAEs led to treatment discontinuation in 11.3 % (n = 8) of patients in investigational arm and 3.1 % (n = 2) in placebo arm. Although pamiparib did not meet its primary endpoint in this study, it showed a favorable safety profile consistent with that of other PARP inhibitors and no new safety signals were detected.
Biomarker relevance in second line therapy of GC
In the phase III GOLD study, the combination olaparib plus paclitaxel missed its primary endpoint – improvement of OS – compared to paclitaxel alone for the treatment of patients with gastric and gastroesophageal junction tumors, who progressed following the frontline therapy [10]. The PARP inhibitor olaparib not only induce DNA damage and cell death but is also involved in the upregulation of PD-L1. Based on these findings, the authors hypothesize that the combination of a cytotoxic chemotherapy (paclitaxel) plus a PARP inhibitor (olaparib) plus an immune checkpoint inhibitor (durvalumab) might potentiate the antitumor activity in gastric cancer [11]. Therefore, a biomarker-oriented study was designed to explore the changes of tumor environment and to evaluate if the anti-PD-L1 addition might enhance the antitumor activity.
An ongoing phase II trial (NCT03579784) is enrolling patients with measurable lesions and histologically confirmed unresectable GC, who have failed to one prior chemotherapy. Patients previously exposed to anti-PD(L1)-1 or PARP inhibitors are excluded. Following the administration schema described on Figure 2, patients receive olaparib (150 mg twice daily on Day 1-28) plus paclitaxel (80 mg/m2 intravenously, IV, on Day 1/8/15) for four cycles, with the addition of durvalumab (1.5g IV on Day 1) on cycles 2 to 4. At the time of progression, biopsy is mandatory; additionally, blood samples for biomarker analysis are collected at each treatment cycle. The DCR per RECIST v1.1 is the primary study endpoint, while the key secondary endpoints include ORR, PFS, OS, quality of life (QoL) and safety. This trial is intended to recruit 40 patients in Korea.
Figure 2: Study design of durvalumab combined to olaparib and paclitaxel
Adjuvant therapy in patients with resected GEA
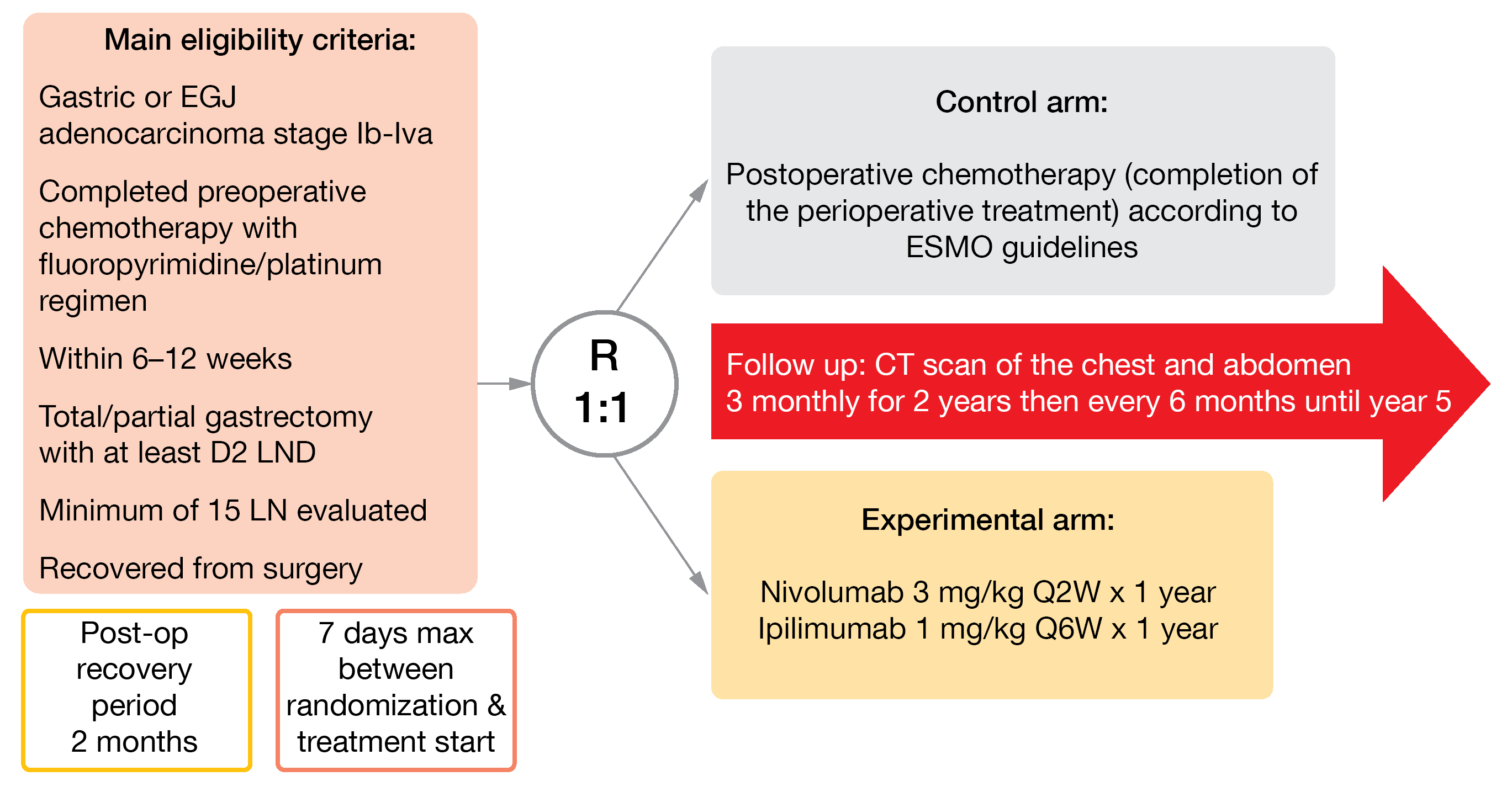
Esophageal cancer is the sixth most common cause of cancer mortality globally; together, gastric and esophageal cancers were responsible for more than 1.3 million deaths in 2020 [1]. In patients with resectable gastroesophageal carcinoma (GEA), ESMO guidelines recommend a neoadjuvant chemotherapy as standard of care [12, 13], which might also be further administered after surgery; however, this regimen leads to suboptimal outcomes. In the CheckMate 577 study, adjuvant immunotherapy with nivolumab – an anti-PD-1 (programmed cell death protein 1) – has proven to be efficient in poor risk patients with GEA treated with neoadjuvant chemotherapy [14]. Moreover, nivolumab and ipilimumab – a CTLA-4 inhibitor – combined therapy showed antitumoral activity in advanced GEA. High risk patients with GEA are defined as those presenting metastatic lymph nodes or a microscopically incomplete surgical resection (R1). According to the data previously presented, high risk GEA patients treated with this dual adjuvant immunotherapy post-resection might have a better disease-free survival (DFS) than those who received standard post-operative chemotherapy [15].
In the international, open-label, randomized, phase II EORTC VESTIGE study (NCT03443856), nivolumab plus ipilimumab versus chemotherapy are currently investigated as adjuvant therapy in high-risk GEA patients [16]. After a post-surgery recovery period of two months, eligible patients are randomized 1:1 to receive for a year either nivolumab (3 mg/kg IV, biweekly) plus ipilimumab (1 mg/kg IV, every six weeks) or the same chemotherapy regimen as pre-operatively (Figure 3). DFS will be primarily analyzed, and OS, safety, toxicity, and QoL secondarily evaluated. As the recruitment opened in August 2019, 95 out of 240 planned patients have been already enrolled until May 2021 in 22 sites in Europe and Israel.
Figure 3: Design of the EORTC 1707 VESTIGE trial
REFERENCES
- Sung H et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71(3): 209-249.
- Cheol Chung Y et al., LEAP-005: A phase 2 multicohort study of lenvatinib plus pembrolizumab in patients with previously treated selected solid tumors—Results from the gastric cancer cohort. J Clin Oncol 2021; 39(suppl 15; abstr 4030).
- van Wilpe S et al., Homologous Recombination Repair Deficiency and Implications for Tumor Immunogenicity. Cancers (Basel) 2021; 13(9).
- Swisher EM et al., Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol 2017; 18(1): 75-87.
- Mirza MR et al., Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med 2016; 375(22): 2154-2164.
- Coleman RL et al., Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390(10106): 1949-1961.
- Xiong Y et al., Pamiparib is a potent and selective PARP inhibitor with unique potential for the treatment of brain tumor. Neoplasia 2020; 22(9): 431-440.
- Xu B et al., Pamiparib dose escalation in Chinese patients with non-mucinous high-grade ovarian cancer or advanced triple-negative breast cancer. Cancer Med 2021; 10(1): 109-118.
- Ciardiello F et al., PARALLEL 303: Phase 2 randomized study of pamiparib vs placebo as maintenance therapy in patients (pts) with inoperable locally advanced or metastatic gastric cancer that responded to platinum-based first-line (1L) chemotherapy. J Clin Oncol 2021; 39(suppl 15; abstr 3109).
- Bang YJ et al., Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18(12): 1637-1651.
- Kim T et al., Biomarker-oriented study of durvalumab in combination with olaparib and paclitaxel in gastric cancer: A phase 2 trial-in-progress. J Clin Oncol 39 2021; 39(suppl 15; abstr TPS4153).
- Lordick F et al., Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27(suppl 5): v50-v57.
- Smyth EC et al., Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27(suppl 5): v38-v49.
- Kelly R et al., Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer (EC/GEJC) following neoadjuvant chemoradiation therapy (CRT): first results of the CheckMate 577 study. Ann Oncol 2020; 31(suppl 4: S1142-S1215).
- Smyth EC et al., Checkpoint inhibitors for gastroesophageal cancers: dissecting heterogeneity to better understand their role in first-line and adjuvant therapy. Ann Oncol 2021; 32(5): 590-599.
- Smyth E et al., EORTC 1707 VESTIGE: Adjuvant immunotherapy in patients with resected gastric cancer following preoperative chemotherapy with high risk for recurrence (ypN+ and/or R1)—An open-label randomized controlled phase II study. J Clin Oncol 2021; 39(suppl 15; abstr TPS4156).
© 2021 Springer-Verlag GmbH, Impressum
More posts
Early insights for CPI combinations in solid tumors
mmunotherapy using anti-PD-1 immune checkpoint inhibitors (CPIs), which is a major therapeutic option in oncology, can potentially achieve synergistic effects once combined with targeted therapies. Drugs targeting proangiogenic factors, such as VEGF and angiopoietin 2 (ANG2), can improve therapeutic responsiveness through their immunosuppressive activity in the tumor environment.
Anti-PD-1 compounds targeting MSI-H/dMMR tumors
Accurate and timely repair of DNA is essential for maintaining genetic stability. Microsatellites are repetitive DNA sequences and particularly prone to replication errors that are normally repaired by the mismatch repair system. Mismatch repair-deficient tumors (dMMR) harbor many mutations in microsatellites, resulting in high levels of microsatellite instability (MSI-H).
PARP- and anti-PD-1-based strategies in breast and cervical cancer
Breast cancer (BC) is the most diagnosed cancer in women and the leading cause of cancer death in females. It has been recently shown that approximately 38 % of female patients younger than 40 years presenting with triple-negative breast carcinomas (TNBC) harbored a germline mutation in breast cancer (BC) susceptibility genes 1 or 2 (gBRCA1/2m).
Checkpoint inhibition: predictors, resistance and immunogenomic features
Immune checkpoints, such as cytotoxic T-lymphocyte associated protein-4 (CTLA-4) or programmed cell death protein 1 (PD-1), downregulate T-cell responses and are crucial for self-tolerance, which protects the body against attacking cells indiscriminately. Tumor cells hijack this mechanism to evade the immune system through the activation of immune checkpoints and inhibition of the T-cell response.
Novel approaches in gastric cancer
With more than 1 million newly diagnosed cases in 2020, gastric cancer was at the fifth place (5.6 %) of the most frequent malignant diseases and accounted for nearly 8 % of cancer deaths worldwide. The multicohort, non-randomized, open-label, phase II LEAP-005 study (NCT03797326) was designed to evaluate the safety and efficacy of a combination – the anti-angiogenic multikinase inhibitor lenvatinib plus the anti-PD-1 antibody pembrolizumab – in patients with previously treated advanced solid tumors.
Preface ASCO Solid Tumor 2021
Because of the ongoing COVID-19 pandemic, this year’s ASCO scientific meeting took place for the second time virtually from Friday, June 04, through Monday, June 08. During these five-day world’s largest oncology conference, approximately 30,000 professionals attended online at least one of the 150 on-demand and broadcast sessions featuring over almost 5,000 abstracts, more than 2,000 poster presentations, 19 oral and 16 educational sessions, as well as opening and plenary sessions, award lectures, cancer-specific highlights sessions, and several clinical cancer symposia.