Clinical findings with sundry targets in various B-cell malignancies
AUGMENT: 5-year results
Recurrent follicular lymphoma (FL) and marginal zone lymphoma (MZL) are treated similarly, mostly with single-agent rituximab. In patients with relapsed/refractory FL, the combination of lenalidomide with rituximab (R2) has previously demonstrated promising efficacy [1]. The multicenter, double-blind, randomized, phase III AUGMENT study was initiated to compare time-limited treatment for approximately one year with R2 vs. rituximab plus placebo in patients with FL grade I-IIIa or MZL who had already received ≥ 1 prior systemic chemotherapy, immunotherapy or chemoimmunotherapy but who were not refractory to rituximab. While lenalidomide 20 mg was taken orally on 21 days of twelve 28-day cycles, rituximab was administered on days 1, 8, 15 and 22 in cycle 1, as well as on day 1 in cycles 2–5. Approximately 180 patients were treated in each arm. At the time of the primary analysis, R2, as compared with R-placebo, induced a higher overall response rate (ORR; 78 % vs. 53 %) and complete remission (CR) rate (34 % vs. 18 %) [2]. Leonard et al. reported updated findings from the AUGMENT trial at ASH 2022 [3].
After a median follow-up of 65.9 months, 70 % and 61 % of patients in the R2 and R-placebo arms, respectively, had completed the treatment. R2 continued to demonstrate superior efficacy over R-placebo. Progression-free survival (PFS), which was defined as the primary endpoint, was significantly improved in the experimental arm (median PFS, 27.6 vs. 14.3 months; HR, 0.50; p < 0.0001). For overall survival (OS), the Kaplan-Meier curve separation after five years continued to favor R2, thus providing evidence for a survival benefit of the combination. Median OS had not been reached in either arm, but the OS rates amounted to 83.2 % vs. 77.3 % at 5 years (HR, 0.59; p = 0.0285).
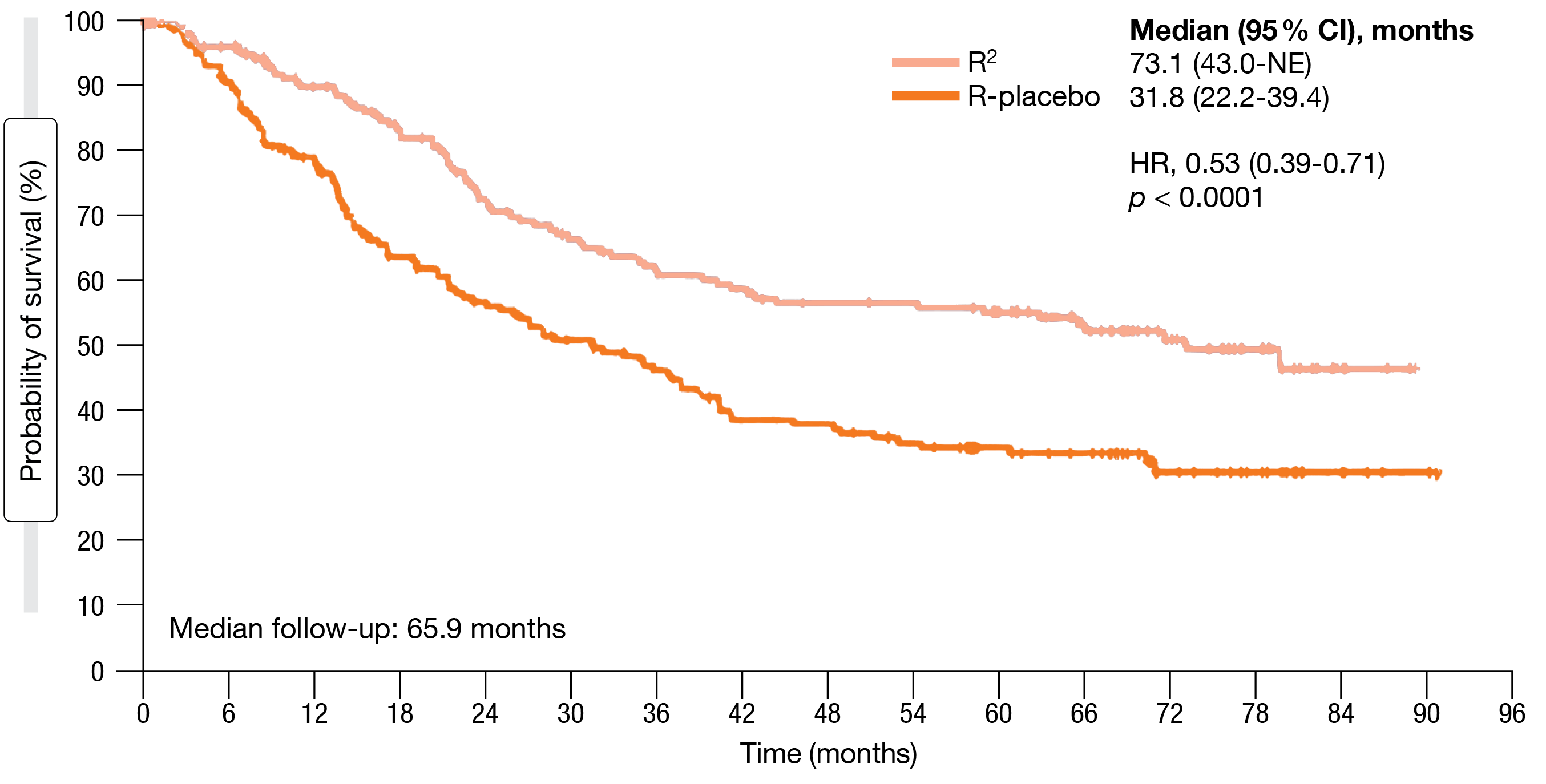
Notably, fewer R2-treated patients required ≥ 1 subsequent therapy (41 % vs. 61 %), with rituximab being the most common agent (25 % and 34 %, respectively). Median time to next lymphoma treatment was 73.1 vs. 31.8 months (HR, 0.53; p < 0.0001; Figure 1), which implied that the treatment-free period extended well beyond the 1-year active treatment period in the experimental arm.
Figure 1: Time to next lymphoma treatment for R2 vs. rituximab plus placebo
Lower rates of SPMs and histologic transformation
The safety profiles remained consistent with the primary analysis. Grade 3/4 treatment-emergent adverse events (TEAEs) were observed more commonly in the R2-treated population (69 % vs. 32 %), with neutropenia occurring most frequently (50 % vs. 13 %). Not surprisingly, patients on lenalidomide treatment were more likely to require dose reductions (26 % vs. 3 % of placebo dose reductions). Also, a greater proportion of patients had dose interruptions with lenalidomide (64 %) vs. placebo (26 %) or rituximab (34 %).
Compared to historical experience, the rates of second primary malignancies (SPMs) and histologic transformations continued to be lower. SPMs occurred in 7 % vs. 12 % of patients treated with R2 and R-placebo, which represented an increase from 3 % and 6 %, respectively, in the primary analysis [2]. The incidence rate of SPMs per 100 patient-years was lower with R2 than with rituximab alone (1,62 % vs. 2.66 %). Six vs. 8 % of patients developed histologic transformations, which resulted in a lower updated incidence rate in the R2 arm (1.24 % vs. 1.85 % per 100 patient-years).
Overall, fewer patients treated with R2 died (15 % vs. 26 %), with most deaths occurring off treatment. Disease progression or disease complication was more commonly the cause of death in the control arm (14 %) than in the experimental arm (5 %). In their summary, the authors pointed out that these updated results, including the OS data, further support the use of the R2 regimen as a standard of care for patients with relapsed/refractory indolent non-Hodgkin lymphoma (NHL).
Zandelisib plus zanubrutinib
The PI3Kδ inhibitor zandelisib has shown durable responses in patients with relapsed/refractory FL in two studies [4, 5]. Preclinical data suggest that dual inhibition of PI3Kδ and BTK results in synergistic efficacy [6]. At ASH 2022, Soumerai et al. presented results from an open-label, multi-arm, phase IB study for the combination of zandelisib with the selective next-generation BTK inhibitor zanubrutinib [7]. The study population consisted of patients with relapsed/refractory FL Grade I-IIIA (n = 31) or relapsed/refractory mantle cell lymphoma (MCL; n = 19) after ≥ 1 prior treatment line. Zandelisib was administered at a daily dose of 60 mg on an intermittent dosing schedule (i.e., days 1–7 of each 28-day cycle) starting from cycle 1 in addition to zanubrutinib 80 mg BID. Intermittent dosing of zandelisib has been shown to improve tolerability, particularly in terms of immune-related AEs, without compromising efficacy [4, 5].
In the group with FL, 86.7 % of patients responded, with 23.3 % and 63.3 % developing CR and partial remission (PR), respectively. Responses lasted for a median of 20.6 months, and median PFS was 22.4 months. Likewise, patients with MCL experienced pronounced and durable responses. The ORR in this group was 72.2 %, and 27.8 % developed CR. Median duration of response had not been reached at the time of the analysis, while median PFS was 10.1 months. Tumor reduction was observed in almost all patients who underwent post-baseline disease assessment in both cohorts. Deepening of responses occurred over time, which calls for longer follow-up to estimate the true CR rates.
The combination of zandelisib and zanubrutinib was tolerated well without an increase in the rate or severity of class-related AEs. TEAEs in the total population most commonly included diarrhea, decreased blood cell counts, and increased blood creatinine levels. Most events were grade 1 and 2, although grade-4 neutrophil count decreases occurred in 12 %. Only 2 patients (4 %) discontinued treatment due to treatment-related TEAEs. One patient died due to COVID-19-related complications. The analysis showed low rates of AEs of special interest; here, transaminase elevations were most common (8.0 %). As the authors concluded, these encouraging preliminary findings support further evaluation of zandelisib plus zanubrutinib in relapsed/refractory FL, MCL and other B-cell malignancies.
Zanubrutinib after acalabrutinib intolerance
Compared to ibrutinib and acalabrutinib, zanubrutinib has shown higher kinase selectivity, which is thought to improve tolerability as many AEs of the less selective BTK inhibitors are potentially caused by off-target inhibition. In the ongoing phase II BGB-3111-215 study, zanubrutinib 160 mg BID or 320 QD was well tolerated in patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), MCL, MZL, or Waldenström’s macroglobulinemia (WM) intolerant to their prior BTK inhibitor therapy [8]. At ASH 2022, Shadman et al. reported an update for cohort 2 of the study that included 21 patients intolerant to acalabrutinib [9]. The primary endpoint was the safety of zanubrutinib as assessed by the recurrence and change in severity of the patients’ acalabrutinib intolerance AEs.
After a median zanubrutinib exposure of 7.6 months, which was longer than the reported cumulative acalabrutinib exposure before discontinuation (4.6 months), 67 % of patients had not experienced any recurrence of their prior acalabrutinib intolerance events. Among the acalabrutinib intolerance events, 75 % did not recur on zanubrutinib at any grade, and none recurred at higher severity. The most common grade ≥ 3 AE was neutrophil count decrease (10 %). In terms of efficacy, zanubrutinib gave rise to an ORR of 61 %, with 94 % of patients achieving at least disease stabilization. According to the authors, these outcomes suggest that patients who are intolerant to acalabrutinib can attain clinical benefit if their treatment is switched to zanubrutinib.
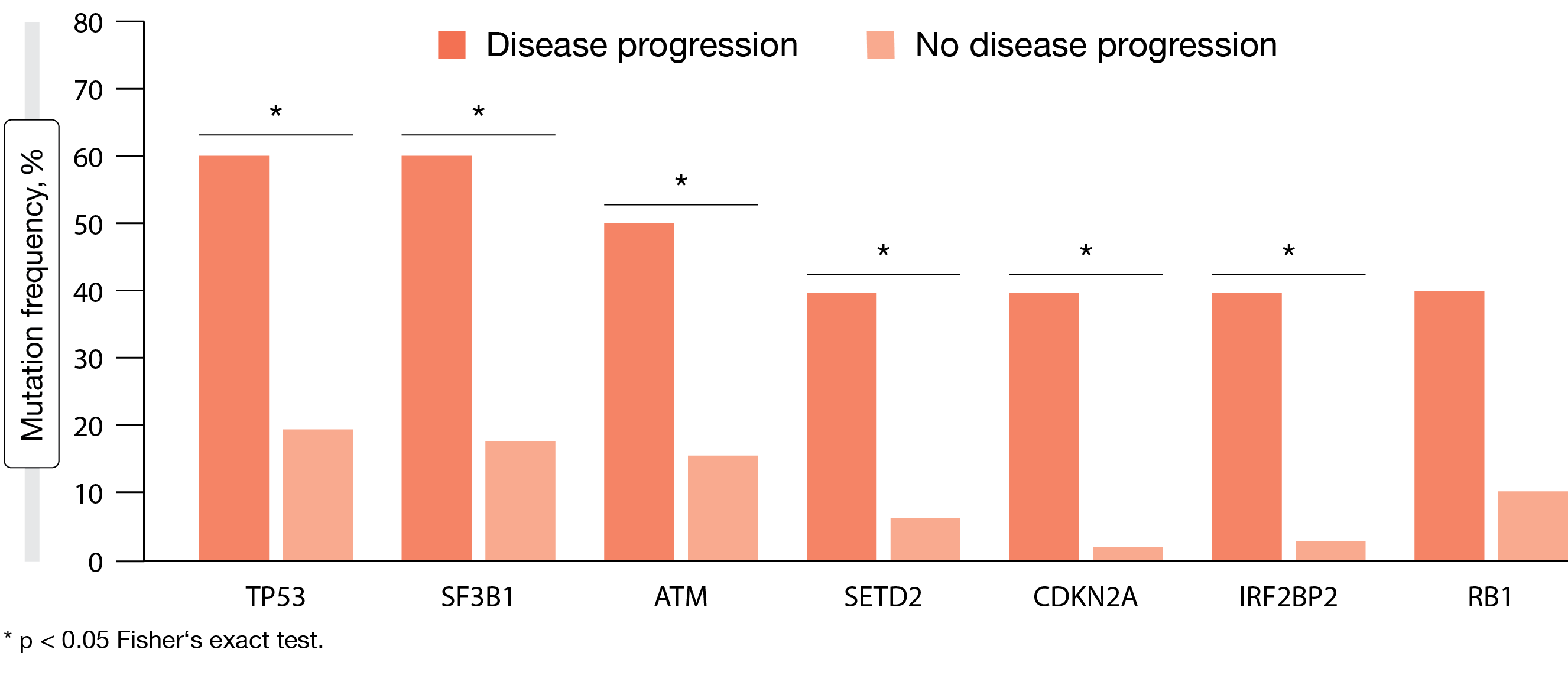
Moreover, Xu et al. evaluated blood samples from 71 patients included in the BGB-3111-215 study to assess the mutational landscape of patients intolerant to BTK inhibitors [10]. This exploratory analysis suggested that mutations of cell cycle, DNA damage, and NOTCH1 pathway genes are frequent in patients intolerant to ibrutinib and/or acalabrutinib. The top mutated genes were TP53 (32 %), SF3B1 (23 %), ATM (18 %), NOTCH1 (17 %), and CHEK2 (15 %). Baseline alterations in cell cycle/DNA damage and epigenetic modifier pathways were associated with inferior response to BTK inhibition (Figure 2) and inferior PFS. Patients who progressed on zanubrutinib showed higher likelihood of BTK mutations that convey resistance to BTK inhibitors or other mutations known to be associated with poor prognosis.
Figure 2: Exploratory analysis: correlation between the presence of baseline genetic alterations and disease progression
Findings in Japanese patients
In the ongoing, multicenter, phase I/II BGB-3111-111 study, zanubrutinib is being investigated in Japanese patients with mature B-cell malignancies including WM, CLL/SLL, relapsed/refractory MCL, FL, and MZL. Part 1 of the study was dedicated to confirming the safety, tolerability, and pharmacokinetics of zanubrutinib, while part 2 evaluated efficacy and safety in disease-specific cohorts.
Investigator-assessed data presented at ASH 2022 for a total of 53 patients [11] indicated that the plasma exposure was comparable to exposures observed in published global zanubrutinib trials at equivalent doses [12-16]. Moreover, the efficacy data were comparable with those observed in these studies. Zanubrutinib was shown to be highly active in Japanese patients. Across the disease-specific cohorts, the ORRs ranged from 82 % to 100 %. Median duration of response and median PFS had not been reached for any cohort.
The treatment was generally well tolerated. Platelet count decreases represented the most common any-grade AE, followed by pyrexia and neutrophil count decreases. Neutropenia and infections were the most common grade ≥ 3 AEs of special interest. Overall, these preliminary data support the use of zanubrutinib in Japanese patients with WM, CLL/SLL, and relapsed/refractory MCL.
Biomarker analysis of zanubrutinib and tislelizumab
Zanubrutinib in combination with the anti-PD-1 antibody tislelizumab has shown antitumor activity in B-cell malignancies, including DLBCL [17, 18]. In the phase I dose-escalation/dose-expansion BGB-3111-A317 study, zanubrutinib plus tislelizumab is being assessed in patients with various relapsed/refractory B-cell malignancies. Lyu et al. performed an exploratory analysis based on samples from 24 patients with DLBCL included in BGB-3111-A317 to identify biomarkers associated with response and resistance to the combination therapy [19].
According to the preliminary results, patients with PD-L1 gene amplification, PD-L1–positive tumor cells, and high mRNA levels of CD3D, HLA-DRA, and LAG3 in baseline tumor tissue might be more responsive to zanubrutinib plus tislelizumab. Enrichment of CD3D, HLA-DRA, and LAG3 suggests an inflamed tumor microenvironment (TME). Non-responders, on the other hand, had high mRNA levels of REL, which were associated with poor clinical outcomes, and higher occurrence of TP53 mutations. The authors noted that the inhibition of B-cell and BCR-related signatures and induction of NK signatures in tumor samples that were observed on zanubrutinib treatment indicate the on-target effect of this agent and its potential TME-modulating effect. However, due to the limited number of samples, these findings must be interpreted with caution.
Zilovertamab plus ibrutinib
The anti-ROR1 antibody zilovertamab has been designed to inhibit signaling related to ROR1, an onco-embryonic kinase-like receptor that is highly expressed in many solid and hematologic malignancies, but not in normal adult tissues [20]. Zilovertamab has been shown to inhibit expression of downstream genes that promote the survival and growth of CLL cells with mutated TP53 in patients treated with BTK inhibitors.
The phase I/II study reported at ASH 2022 by Lee et al. demonstrated safety and robust efficacy of the combined use of zilovertamab and ibrutinib in patients with relapsed/refractory MCL and treatment-naïve or relapsed/refractory CLL [21]. Parts 1 and 2 of the study were dedicated to dose finding and dose expansion; here, patients with CLL (n = 34) and MCL (n = 33) were treated with zilovertamab plus ibrutinib, while an MZL cohort was still enrolling at the time of the analysis. The recommended phase II regimen included 3 doses of zilovertamab 600 mg Q2W followed by 600 mg Q4W plus the approved doses of ibrutinib for the treatment of CLL or MCL. In the third part of the study, zilovertamab plus ibrutinib was tested against ibrutinib monotherapy in patients with CLL. Eighteen individuals received the combination, while 10 were treated with single-agent ibrutinib. Overall, this was a heavily pretreated population with high-risk disease.
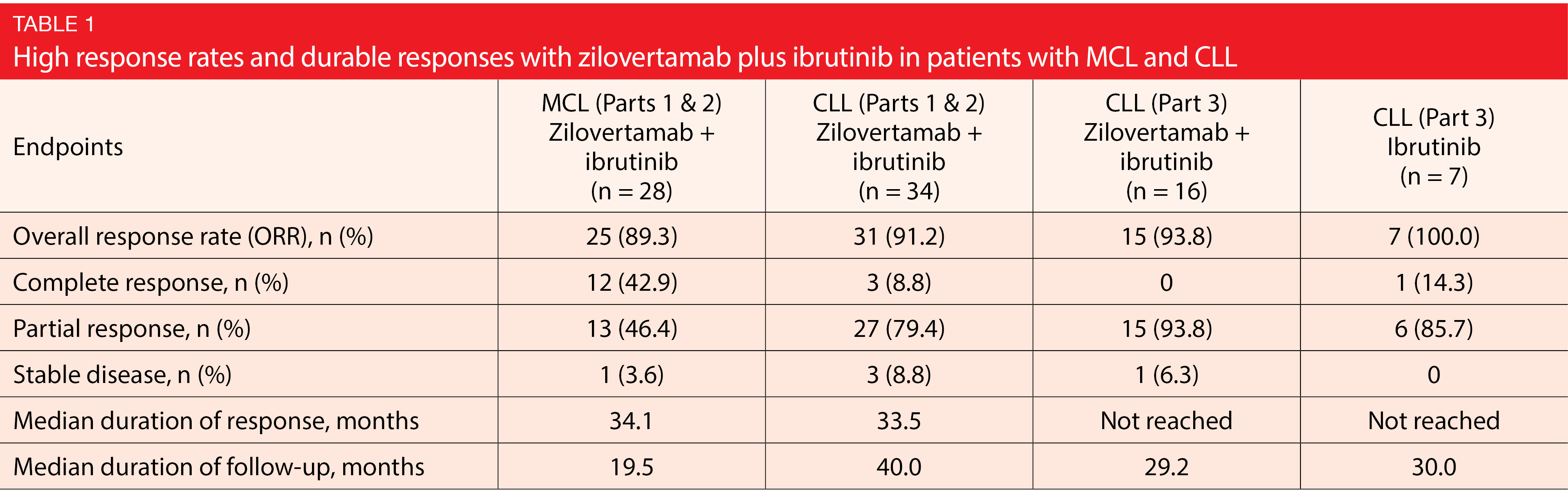
Across the study parts, zilovertamab plus ibrutinib gave rise to robust and durable responses (Table 1). ORRs ranged from 89.3 % to 100.0 %. Median duration of response was almost 3 years in the MCL and CLL cohorts, while it had not been reached yet by either treatment arm in the randomized comparison. The safety profile of the combination was consistent with that of ibrutinib monotherapy. Diarrhea and fatigue occurred most often, and grade 3/4 events were rare. Atrial fibrillation was seen in 9.4 % of patients. The majority of laboratory abnormalities was grade 1 or 2. In part 3 of the study, the combination induced grade 3/4 decreases in neutrophil counts in 5.6 %, while this rate was much higher in the ibrutinib-only arm (20.0 %). Febrile neutropenia was observed in 1.2 % of patients treatment with zilovertamab plus ibrutinib.
Activity for MCL and CLL
The scientists analyzed the efficacy of zilovertamab plus ibrutinib separately for MCL patients and CLL patients. In the MCL population, the combination led to rapid responses, with CR and PR rates of 17.9 % and 60.7 %, respectively, already at month 3. At 26 months, the ORR and CR rate were 89.3 % and 42.9 %, respectively. In contrast, historical ibrutinib has given rise to response rates that plateaued at a markedly lower level after increasing slowly over the course of one year [22].
Also, the combination elicited favorable PFS compared to ibrutinib alone, as > 70 % of MCL patients were progression-free at 24 months. Analyses of PFS by prior line of therapy demonstrated durable results maintained in the second line that again exceeded the historical findings shown for ibrutinib [23]. Moreover, zilovertamab plus ibrutinib displayed robust efficacy with regard to PFS across subgroups with poor prognosis including patients with Ki-67 % index ≥ 30 % (median PFS, 33.21 months) and TP53 mutation (median PFS, not reached). Median OS in the total MCL cohort had not been reached after a median follow-up of 19.5 months; at 25 months, 70 % of patients were alive. Historical ibrutinib has given rise to a median OS of 25.0 months in the same setting [22]. Based on these data, the global, placebo-controlled phase III ZILO-301 trial investigating ibrutinib with or without zilovertamab has been initiated in patients with relapsed/refractory MCL (NCT05431179).
In the group with CLL, the median PFS for zilovertamab plus ibrutinib had not been reached after a median follow-up of 40 months. At 24 months, 95 % of patients were progression-free. For reference, the ALPINE study showed 24-month PFS rates of 79.5 % and 67.3 % for zanubrutinib and ibrutinib, respectively, in patients with relapsed/refractory CLL [24]. The pooled landmark analysis for CLL patients with TP53 mutation/17p deletion across all study parts yielded encouraging findings, as the entire group remained progression-free at 42 months.
BGB-11417 monotherapy
Although the efficacy of Bcl-2 inhibitors in CLL/SLL has been established by the approval of agents such as venetoclax across all lines of therapy, AEs and the development of BCL2 mutations leading to resistance limit the utility of this treatment in clinical practice [25, 26]. BGB-11417 has been developed as a more potent and highly selective Bcl-2 inhibitor than venetoclax, with the potential to achieve deeper target inhibition and clinical responses [27, 28]. According to preliminary data obtained from the phase I BGB-11417-102 study, BGB-11417 monotherapy is well tolerated at all tested doses of up to 640 mg/d and shows promising efficacy in patients with mature B-cell malignancies [29]. Escalating doses with a weekly ramp-up for CLL/SLL (n = 18) and a daily ramp-up for NHL (i.e., FL, MZL, DLBCL or TF-NHL; n = 30) were investigated in the relapsed/refractory setting.
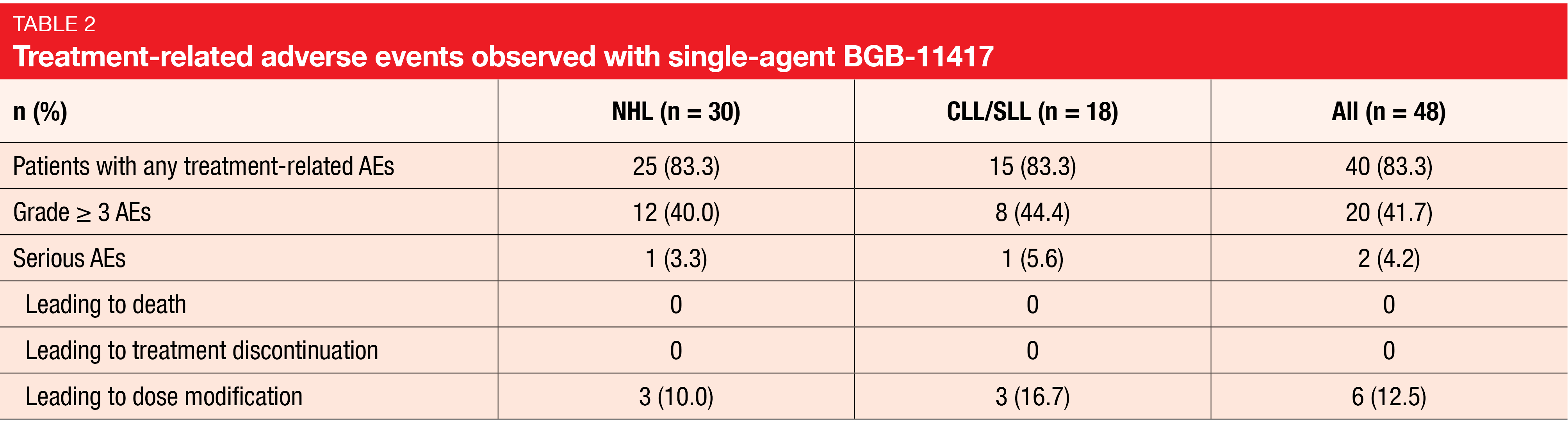
At the time of the analysis, the majority of treatment-related AEs were grade 1/2 events. No treatment-related AEs led to treatment discontinuation or death for both CLL/SLL and NHL patients (Table 2). The most common grade ≥ 3 TEAE was decreased neutrophil counts (22.9 %), although this was transient and controllable. None of the patients developed clinical TLS. The data did not suggest any dose-dependent toxicity increases.
Among the 10 patients with CLL/SLL who were available for tumor assessment, 6 responded already at the lower dose levels. At the 80 mg/d dose level, 2 and 1 obtained CR and PR, respectively, while 3 developed PR at the 160 mg/d dose level. Owing to the short follow-up, no response data were available for the 320 mg/d and 640 mg/d cohorts yet. Two out of 7 patients assessable for MRD negativity achieved undetectable MRD. Preliminary activity was noted in the NHL patients, with 5 responding in the group of 25 individuals available for assessment. Further expansion data are being generated here, as the authors emphasized. Dose escalation is still ongoing, and the maximum tolerated dose/maximum absorbable dose has not been determined to date.
BGB11417 with and without zanubrutinib
Preliminary results from the first-in-human, phase I, dose-escalation and dose-expansion BGB-11417-101 study were presented by Soumerai et al. for patients with relapsed/refractory NHL or WM who received BGB-11417 either as a single agent (parts 1 and 2) or in combination with zanubrutinib (parts 3 and 4) [30]. In the total group of 59 individuals, 43 and 16 received the monotherapy and the combination, respectively. NHL included DLBCL, FL, MZL, and MCL. Only patients with MCL were treated with the combination.
BGB-11417 was shown to be tolerable at doses of up to 640 mg/d. In the group with NHL that was treated with single-agent BGB-11417, only 1 dose-limiting toxicity of febrile neutropenia occurred, and the maximum tolerated dose was not reached. BGB-11417 plus zanubrutinib was also well tolerated at doses of BGB-11417 ≤ 320 mg; here, dose escalation is ongoing in patients with MCL. No clinical TLS emerged. Gastrointestinal AEs represented the most common monotherapy toxicity, although all cases were mild. Likewise, neutropenic events, which were the most common AE with the combined regimen, typically remained in the grade 1/2 range.
Responses occurred in 43 % of WM patients and 10 % of NHL patients treated with the monotherapy; in the group that received BGB-11417 plus zanubrutinib, the ORR was 81 %. Complete remissions resulted in 67 % of the patients who received the combination. Taken together, these data demonstrate the efficacy of BGB-11417 monotherapy in NHL and WM as well as that of BGB-11417 plus zanubrutinib in MCL. The study continues to determine the recommended phase II dose in the monotherapy and combination therapy settings.
REFERENCES
- Leonard JP et al., Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401
(Alliance). J Clin Oncol 2015; 33(31): 3635-3634 - Leonard JP et al., AUGMENT: a phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol 2019; 37(14): 1188-1199
- Leonard JP et al., Five-year results and overall survival update from the phase 3 randomized study AUGMENT: lenalidomide plus rituximab versus rituximab plus placebo in patients with relapsed/refractory indolent non-Hodgkin lymphoma. ASH 2022,
abstract 230 - Pagel JM et al., Zandelisib with continuous or intermittent dosing as monotherapy or in combination with rituximab in patients with relapsed or refractory B-cell malignancy: a multicentre, first-in-patient, dose-escalation and dose-expansion, phase 1b trial. Lancet Oncol 2022; 23(8): 1021-1031
- Zelenetz AD et al., Efficacy and safety of single-agent zandelisib administered by intermittent dosing in patients with relapsed or refractory follicular lymphoma: final results of the Tidal phase 2 study. ASH 2022, abstract 1563
- de Rooji MFM et al., Ibrutinib and idelalisib synergistically target BCR-controlled adhesion in MCL and CLL: a rationale for combination therapy. Blood 2015; 125(14): 2306-2309
- Soumerai JD et al., Safety and efficacy of the PI3Kδ inhibitor zandelisib in combination with the BTK inhibitor zanubrutinib in patients with relapsed/refractory follicular lymphoma or mantle cell lymphoma. ASH 2022, abstract 78
- Shadman M et al., Zanubrutinib in patients with previously treated B-cell malignancies intolerant of previous Bruton tyrosine kinase inhibitors in the USA: a phase 2, open-label, single arm study. Lancet
Haematol 2023; 10(1): e35-e45 - Shadman M et al., Zanubrutinib in acalabrutinib-intolerant patients with B-cell malignancies. ASH 2022, abstract 1587
- Xu L et al., Genomic characterization of patients in a phase 2 study of zanubrutinib in BTK inhibitor-intolerant patients with relapsed/refractory B-cell
malignancies. ASH 2022, abstract 4176 - Ishikawa T et al., Efficacy and safety of zanubrutinib in Japanese patients with mature B-cell malignancies. ASH 2022, abstract 1590
- Tam CS et al., Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial.
Lancet Oncol 2022, 23(8): 1031-1043 - Hillmen P et al., First interim analysis of ALPINE study: results of a phase 3 randomized study of zanubrutinib vs ibrutinib in patients with relapsed/
refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. EHA 2021, LB1900 - Tam CS et al., A head-to-head Phase III study comparing zanubrutinib versus ibrutinib in patients with Waldenström macroglobulinemia. Future Oncol 2018; 14(22): 2229-2237
- Song Y et al., Zanubrutinib in relapsed/refractory mantle cell lymphoma: long-term efficacy and safety results from a phase 2 study. Blood 2022; 139(21): 3148-3158
- Tam CS et al., Pooled safety analysis of zanubrutinib monotherapy in patients with B-cell malignancies. Blood Adv 2022; 6(4): 1296-1308
- Tam CS et al., An Update on safety and preliminary efficacy of highly specific Bruton tyrosine kinase (BTK) inhibitor zanubrutinib in combination with PD-1 Inhibitor tislelizumab in patients with previously treated B-cell lymphoid malignancies. Blood 2019; 134 (Supplement_1): 1594
- Tislelizumab; National Medical Products Administration, 2022
- Lyu J et al., Biomarker analysis of zanubrutinib and tislelizumab combination therapy in patients with relapsed/refractory B-cell malignancies. ASH 2022, abstract 1529
- Kipps TJ, ROR1: an orphan becomes apparent. Blood 2022; 140(14): 1583-1591
- Lee HJ et al., Phase 1/2 study of zilovertamab and ibrutinib in mantle cell lymphoma, chronic lymphocytic leukemia, or marginal zone lymphoma. ASH 2022, abstract 232
- Rule S et al., Outcomes in 370 patients with mantle cell lymphoma treated with ibrutinib: a pooled analysis from three open-label studies. Br J Haematol 2017; 179(3): 430-438
- Dreyling M et al., Long-term Outcomes With Ibrutinib Treatment for Patients With Relapsed/
Refractory Mantle Cell Lymphoma: A Pooled Analysis of 3 Clinical Trials With Nearly 10 Years of Follow-up. HemaSphere 2022; 6(5): e712 - Brown JR et al., Zanubrutinib demonstrates superior progression-free survival compared with ibrutinib for treatment of relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma: Results from final analysis of ALPINE randomized phase 3 study. ASH 2022, abstract LBA-6
- Davids MS et al., Comprehensive safety analysis of venetoclax monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia.
Clin Cancer Res 2018; 24(18): 4371-4379 - Tausch E et al., Venetoclax resistance and acquired BCL2 mutations in chronic lymphocytic leukemia. Haematologica 2019; 104(9): e434-e437
- Hu N et al., Preclinical characterization of BGB-11417, a potent and selective Bcl-2 inhibitor with
superior antitumor activities in haematological tumor models. Cancer Res 2020; 80(suppl 16): 3077 - Tam CS et al., Preliminary safety and efficacy data from patients with relapsed/ refractory (R/R)
B-cell malignancies treated with the novel B-cell lymphoma 2 (BCL2) inhibitor BGB-11417 in monotherapy or in combination with Zanubrutinib. Blood 2021; 138(suppl 1): 1419 - Li C et al., A phase 1 study evaluating the safety, tolerability, pharmacokinetics, and preliminary antitumor activity of Bcl-2 inhibitor BGB-11417 in adult
patients with mature B-cell malignancies: preliminary data. ASH 2022, abstract 2989 - Soumerai JD et al., A phase I study with the novel B-cell lymphoma 2 inhibitor BGB-11417 as monotherapy or in combination with zanubrutinib in patients with non-Hodgkin lymphoma or Waldenström macroglobulinemia: preliminary data. ASH 2022, abstract 4201
© 2023 Springer-Verlag GmbH, Impressum
More posts
Clinical findings with sundry targets in various B-cell malignancies
Recurrent follicular lymphoma (FL) and marginal zone lymphoma (MZL) are treated similarly, mostly with single-agent rituximab. In patients with relapsed/refractory FL, the combination of lenalidomide with rituximab (R2) has previously demonstrated promising efficacy. The multicenter, double-blind, randomized, phase III AUGMENT study was initiated to compare time-limited treatment for approximately one year with R2 vs. rituximab plus placebo in patients with FL grade I-IIIa or MZL who had already received ≥ 1 prior systemic chemotherapy, immunotherapy or chemoimmunotherapy but who were not refractory to rituximab.
Follicular lymphoma: bispecific and PI3Kδ-targeted approaches
Advanced-stage follicular lymphoma (FL) remains incurable, with most patients eventually experiencing disease progression despite therapeutic advances. Relapsed or refractory FL is challenging to treat, particularly in high-risk patients who are refractory to prior treatments and have progressed within 24 months. The combination of rituximab and lenalidomide (R2) is commonly used in this setting, although complete response (CR) rates are suboptimal.
New approaches in relapsed and refractory DLBCL
Approximately one third of patients with diffuse large B-cell lymphoma (DLBCL) develop relapsed or refractory disease, which remains a major cause of mortality. In patients relapsing more than 1 year after first-line treatment, the standard of care is salvage treatment followed by autologous stem cell transplantation (ASCT), although responses to platinum-based salvage therapy are generally suboptimal.
Chronic lymphocytic leukemia: moving towards new horizons
The first-in-class, covalent BTK inhibitor ibrutinib has transformed the treatment of patients with chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL). However, toxicities frequently lead to treatment discontinuation. Moreover, exposure coverage between dosing intervals falls below the IC50 threshold, and BTK occupancy at trough levels is variable.
Further steps to improve efficacy and safety in acute myeloid leukemia
Venetoclax 400 mg QD in combination with azacitidine 75 mg/m2 on days 1–7 has been approved for the treatment of patients with newly diagnosed acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy based on the phase III VIALE-A trial that met its primary endpoint of overall survival (OS) at the time of the interim analysis conducted in March 2020.
Active monotherapies and combinations in mantle cell lymphoma
The initial treatment of patients with mantle-cell lymphoma (MCL) is continuously evolving due to the introduction of new targeted agents. Ruan et al. conducted a single-arm phase II study based on the hypothesis that the addition of the next-generation BTK inhibitor acalabrutinib to lenalidomide plus rituximab (ALR) would synergize activity and accelerate minimal residual disease (MRD)-negative complete response (CR), thus allowing for response-adapted adjustment of treatment intensity to minimize toxicity.