Final analysis of the MAGNOLIA trial: zanubrutinib in marginal zone lymphoma
The global, open-label, single-arm, phase II MAGNOLIA trial was designed to assess the next-generation BTK inhibitor zanubrutinib in patients with relapsed/refractory marginal zone lymphoma (MZL) who had previously received ≥ 1 CD20-directed regimen. After a median follow-up of 15.7 months, the overall response rate (ORR) according to independent review was 68.2 %, with complete remissions resulting in 25.8 % [1]. Median duration of response and median progression-free survival had not been reached yet. At both 12 and 15 months, 82.5 % of patients were alive and progression-free. Opat et al. presented the final analysis of the MAGNOLIA study at a median follow-up of 28 months at ASH 2022 [2]. The safety and efficacy populations comprised 68 and 66 individuals, respectively. Thirty-one were continuing zanubrutinib treatment on the long-term extension study at the time of the analysis.
Zanubrutinib was generally well tolerated, and no new safety signals emerged. Contusion, diarrhea and constipation were reported as the most common treatment-emergent AEs (TEAEs), with almost all cases rated as mild or moderate. Neutropenia occurred in 16 %; here, 12 % of events were grade ≥ 3. TEAEs of clinical interest mainly included infections (all grades, 56 %; grade ≥ 3, 22 %) and hemorrhage (all grades, 41 %; grade ≥ 3, 1.5 %). Hypertension and atrial fibrillation/flutter were infrequent and showed lower rates than reported for ibrutinib [3]. All-grade second primary malignancies were noted in 7 %.
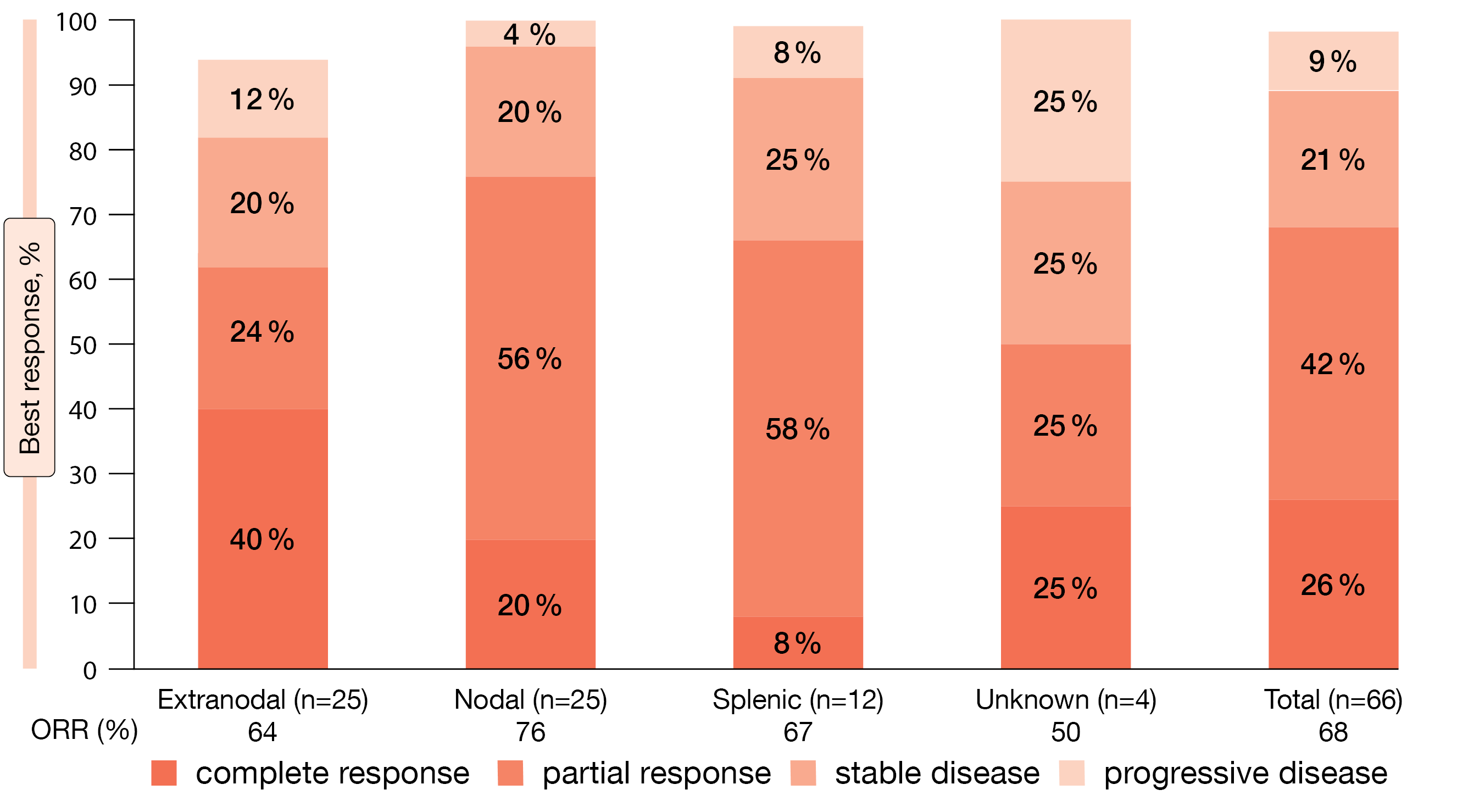
Zanubrutinib treatment gave rise to high response rates and durable disease control. The ORR according to PET and/or CT by independent review committee (i.e., the primary endpoint) was 68 %, with complete responses being achieved in 26 %. Median time to response was relatively short at 2.8 months. According to CT only, 67 % of patients responded. Remissions were seen across MZL subtypes (Figure) as well as regardless of age, disease stage, presence of bone marrow involvement, and prior treatment. Median progression-free survival, median overall survival and median duration of response had not been reached. At 24 months, 86 % of patients in the entire group were alive, and 71 % were progression-free. In 73 % of cases, responses lasted through 2 years. For all of these endpoints, no obvious differences emerged across MZL subtypes.
Figure: Best overall responses across marginal zone lymphoma subtypes by independent review
REFERENCES
- Opat S et al., The MAGNOLIA trial: zanubrutinib, a next-generation Bruton tyrosine kinase inhibitor, demonstrates safety and efficacy in relapsed/refractory marginal zone lymphoma. Clin Cancer Res 2021; 27(23): 6323-6332
- Opat S et al., Long-term efficacy and safety of zanubrutinib in patients with relapsed/refractory marginal zone lymphoma: final analysis of the
MAGNOLIA (BGB-3111-214) trial. ASH 2022, abstract 234 - Tam CS et al., Rate of atrial fibrillation in patients with B-cell malignancies who undergo treatment with zanubrutinib, 2022 Lymphoma, Leukemia and Myeloma Congress, abstract 1324736
© 2023 Springer-Verlag GmbH, Impressum
More posts
Clinical findings with sundry targets in various B-cell malignancies
Recurrent follicular lymphoma (FL) and marginal zone lymphoma (MZL) are treated similarly, mostly with single-agent rituximab. In patients with relapsed/refractory FL, the combination of lenalidomide with rituximab (R2) has previously demonstrated promising efficacy. The multicenter, double-blind, randomized, phase III AUGMENT study was initiated to compare time-limited treatment for approximately one year with R2 vs. rituximab plus placebo in patients with FL grade I-IIIa or MZL who had already received ≥ 1 prior systemic chemotherapy, immunotherapy or chemoimmunotherapy but who were not refractory to rituximab.
Follicular lymphoma: bispecific and PI3Kδ-targeted approaches
Advanced-stage follicular lymphoma (FL) remains incurable, with most patients eventually experiencing disease progression despite therapeutic advances. Relapsed or refractory FL is challenging to treat, particularly in high-risk patients who are refractory to prior treatments and have progressed within 24 months. The combination of rituximab and lenalidomide (R2) is commonly used in this setting, although complete response (CR) rates are suboptimal.
New approaches in relapsed and refractory DLBCL
Approximately one third of patients with diffuse large B-cell lymphoma (DLBCL) develop relapsed or refractory disease, which remains a major cause of mortality. In patients relapsing more than 1 year after first-line treatment, the standard of care is salvage treatment followed by autologous stem cell transplantation (ASCT), although responses to platinum-based salvage therapy are generally suboptimal.
Chronic lymphocytic leukemia: moving towards new horizons
The first-in-class, covalent BTK inhibitor ibrutinib has transformed the treatment of patients with chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL). However, toxicities frequently lead to treatment discontinuation. Moreover, exposure coverage between dosing intervals falls below the IC50 threshold, and BTK occupancy at trough levels is variable.
Further steps to improve efficacy and safety in acute myeloid leukemia
Venetoclax 400 mg QD in combination with azacitidine 75 mg/m2 on days 1–7 has been approved for the treatment of patients with newly diagnosed acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy based on the phase III VIALE-A trial that met its primary endpoint of overall survival (OS) at the time of the interim analysis conducted in March 2020.
Active monotherapies and combinations in mantle cell lymphoma
The initial treatment of patients with mantle-cell lymphoma (MCL) is continuously evolving due to the introduction of new targeted agents. Ruan et al. conducted a single-arm phase II study based on the hypothesis that the addition of the next-generation BTK inhibitor acalabrutinib to lenalidomide plus rituximab (ALR) would synergize activity and accelerate minimal residual disease (MRD)-negative complete response (CR), thus allowing for response-adapted adjustment of treatment intensity to minimize toxicity.