Combinational immunotherapy of nivolumab and ipilimumab prolongs survival in comparison to standard chemotherapy in malignant pleural mesothelioma
CheckMate743, a randomized, open-label, phase III study evaluate the efficacy and safety of the combination treatment of two immunotherapy drugs, nivolumab (NIVO), an anti-PD-1 antibody, and ipilimumab (IPI), an anti-CTL4-A antibody, as compared to standard chemotherapy in patients with malignant pleural mesothelioma (MPM). Patients diagnosed with an unresectable MPM with no prior systemic therapy and good ECOG performance status (0 or 1) were enrolled in the trial. Tumor histology (epithelioid vs non-epithelioid) and gender were two factors used to stratify patients before randomization.
A total of 605 patients were randomized in a 1-to-1 ratio to receive either standard chemotherapy of cisplatin/carboplatin plus pemetrexed for six cycles or immunotherapy combination of NIVO every two weeks plus IPI every six weeks for up to 2 years or until disease progression or unacceptable toxicity. The primary endpoint for the study was overall survival (OS).
The baseline characteristics were similar between both treatment groups, with around 40% of patients belonging to the ECOG performance status 0, 40% never smokers and 75% categorized histologically as epithelioid. About 20% of patients had <1% PD‑L1 expression at baseline, quantified using a PD-L1 immunohistochemistry assay.
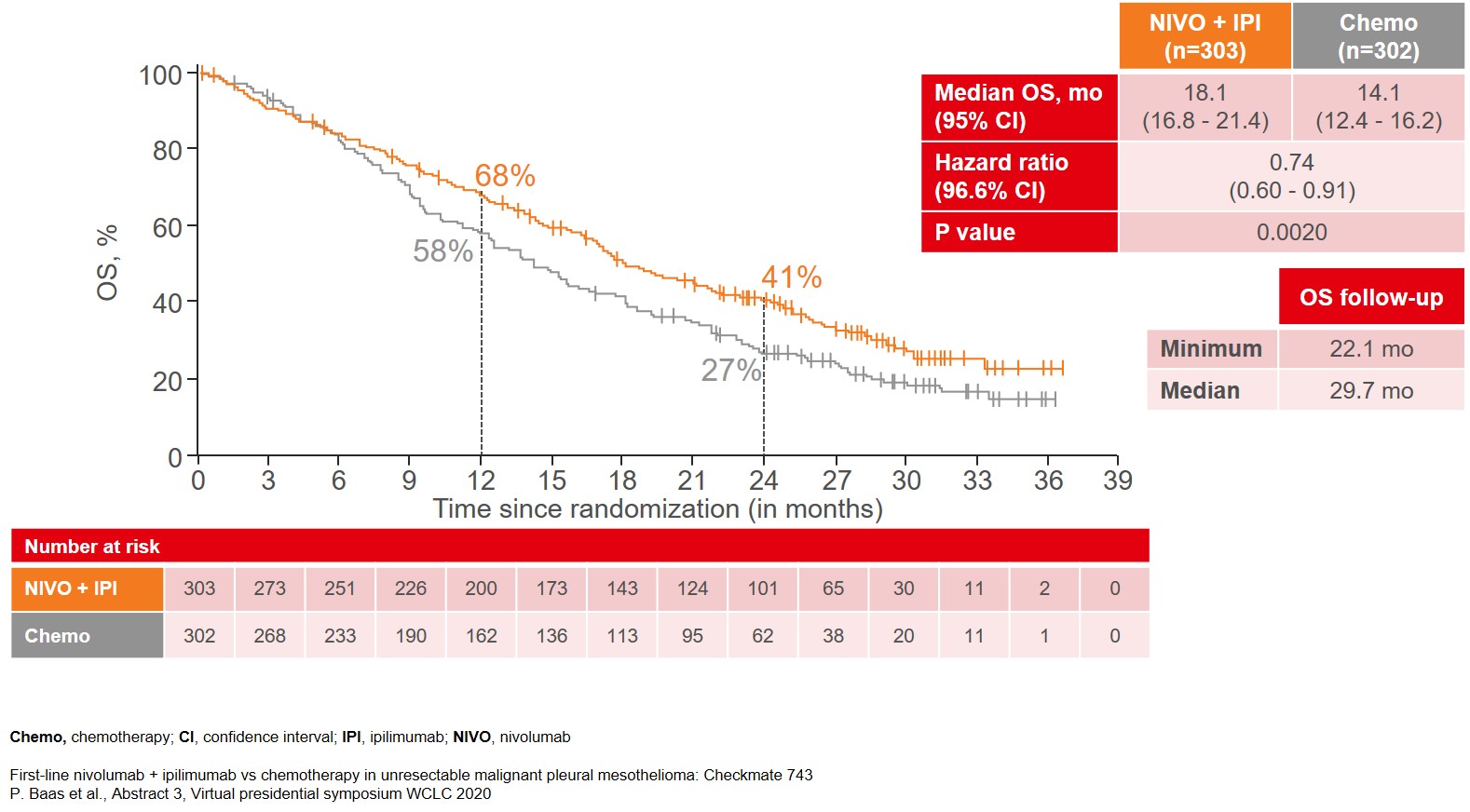
The data from a prespecified interim analysis of CheckMate743, after a median follow-up of 29.7 months, showed that the trial met its primary endpoint with the combination treatment of NIVO and IPI yielding a significant 4-month improvement in median OS as compared to platinum doublet chemotherapy (18.1 vs 14.1 months), after a median follow-up of 29.7 months (Figure 1).
Figure 1: Median Overall Survival (OS) as the primary endpoint of the CheckMate 743 study
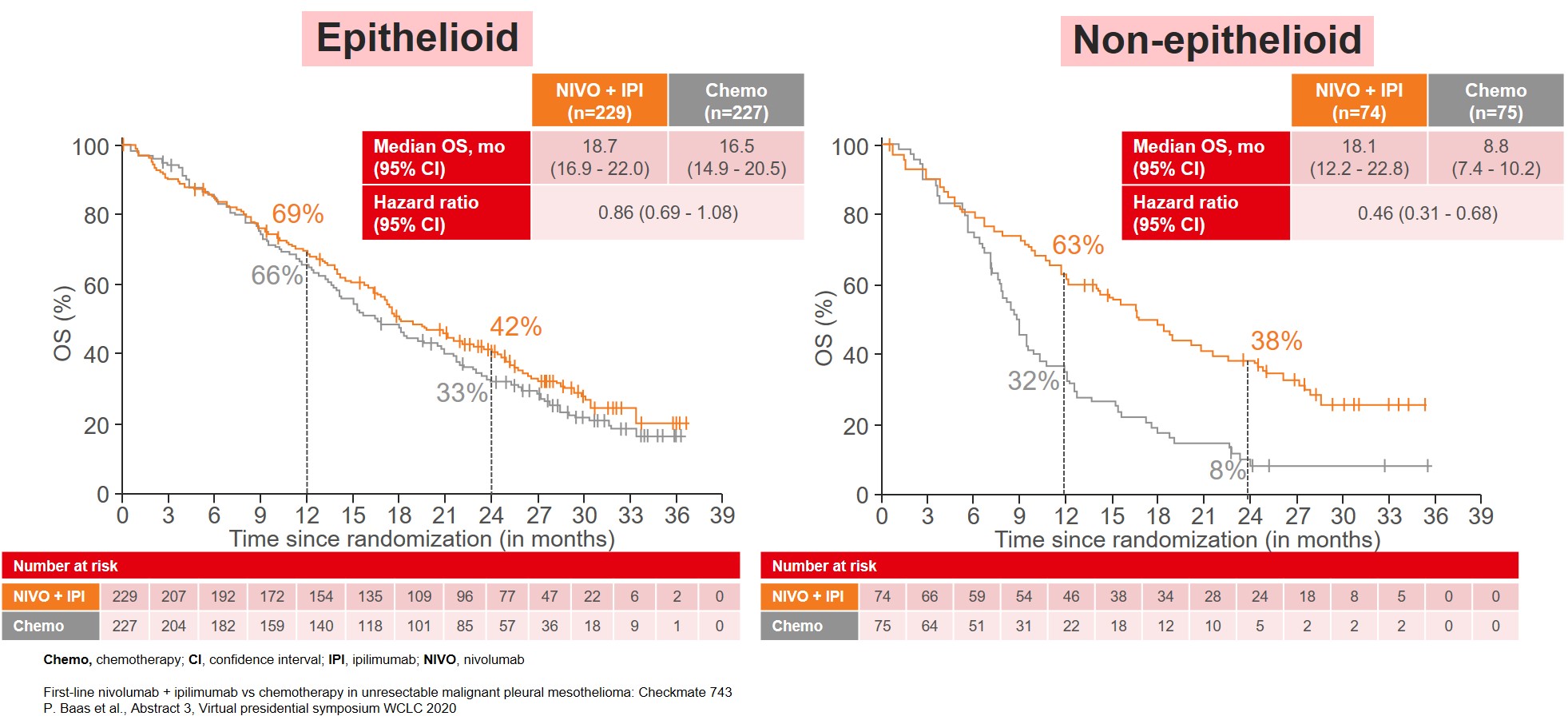
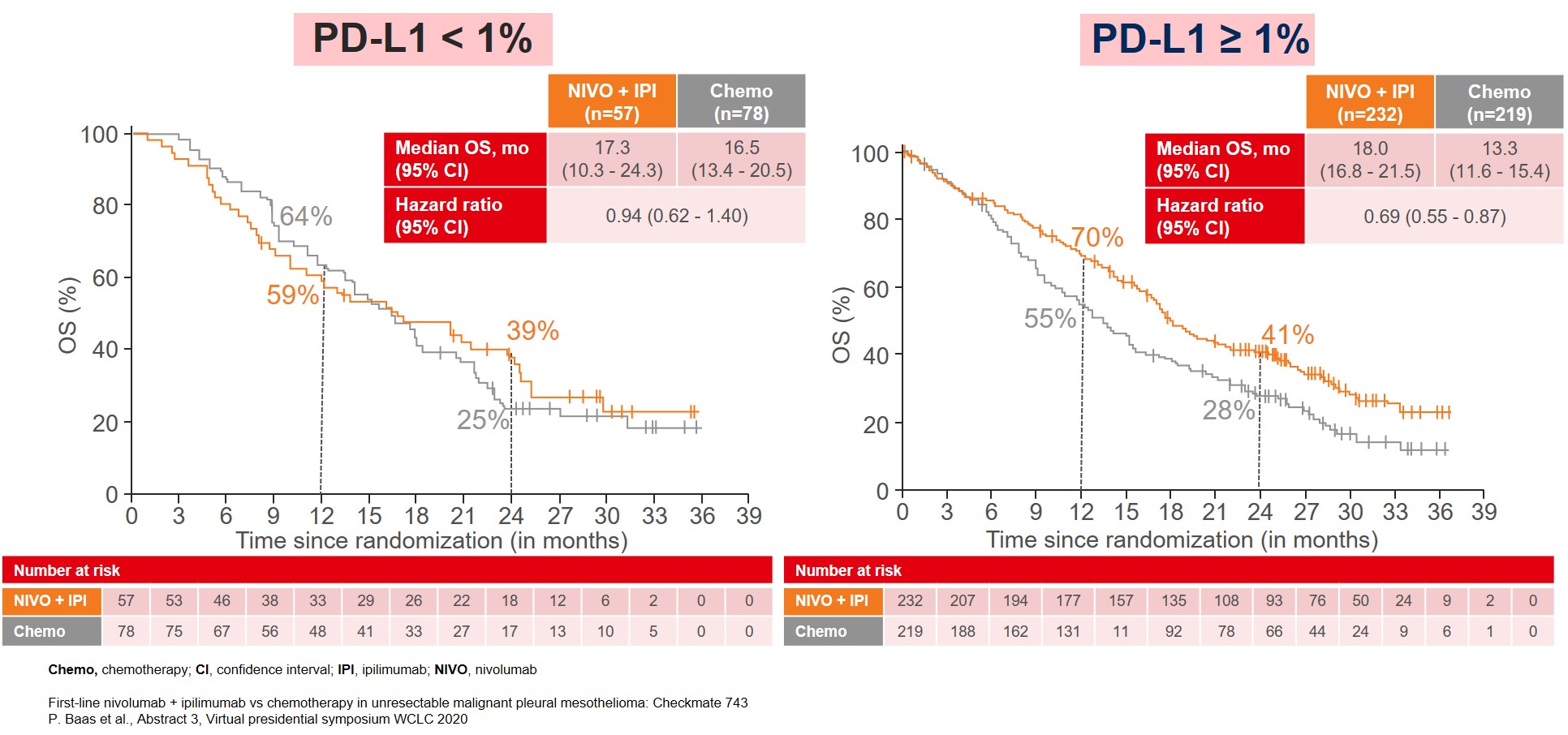
The improvement in OS was consistent among almost all subgroups, especially in patients categorized with non-epithelioid tumor (Figure 2) and patients with PD-L1≥1% (Figure 3).
Figure 2: Median OS by tumor histology
Although the NIVO plus IPI treatment arm showed similar outcomes compared to the standard chemotherapy arm for other efficacy outcomes such as median progression-free survival (mPFS) (6.8 vs 7.2 months, HR 1.00, 95% 0.82 – 1.2) and the objective response rate (ORR) (40% vs 43%), the duration of response (DOR) different, with DOR of 11 months with NIVO plus IPI at 11 months vs 6.7 months with chemotherapy.
Figure 3: Median OS by PD-L1 expression
The adverse event profiles for the two treatment regimens were markedly different but consistent with the AEs previously seen at a similar dose and schedule in other studies with the most common treatment-related adverse events (TRAEs) (≥15%) in the NIVO plus IPI group being diarrhea and pruritis whereas with standard chemotherapy the most common TRAES were anemia, neutropenia, fatigue, decreased appetite and asthenia. The difference in the incidence of serious TRAEs (15% vs 6%) and TRAEs leading to discontinuation of treatment regimen (15% vs 7%) was higher in NIVO plus IPI group compared to chemotherapy.
In conclusion, the results reported from the CheckMate743 trial led to the approval of nivolumab and ipilimumab by the Food and Drug Administration as first-line treatment patients with all types of unresectable malignant pleural mesothelioma across all subgroups1.
REFERENCE
- https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-nivolumab-and-ipilimumab-unresectable-malignant-pleural-mesothelioma
More posts
Interview: Lung cancer screening: hurdles in daily routine and in the research laboratory
Interview: Lung cancer screening: hurdles in daily routine and in the research laboratory