Determination of clinical responses to immunotherapy
In the past 13 years, an earthshaking change has occurred in treatment of non–small-cell lung cancer (NSCLC). The emergence of two new treatment methods – targeted treatment and immunotherapy – has overturned doctors’ and patients’ perception of standard of care for NSCLC. However, not all of the driver oncogenes in NSCLC have been identified. There is still a large number of patients with unknown driver-oncogene mutations who do not benefit from the currently available targeted therapies. Furthermore, acquired resistance to these targeted therapies invariably develops.
The development of immunotherapies is based on three important features of the immune system: specificity, adaptability, and memory. The concept of immunotherapy is based on elimination of cancer cells through regulation of the immune microenvironment or breaking of immunological tolerance, which is different from other conventional treatment concepts. Immunotherapies have been applied for treatment of various cancers, which has shown that they can significantly prolong patient progression-free survival (PFS) and overall survival (OS) for solid tumors. At CSCO 2017, the topics on immunotherapy for NSCLC mainly focused on: 1. First-line and second-line immunotherapies; 2. Determination of responses to immunotherapies; 3. Choice of suitable biomarker(s); and 4. Duration of immunotherapy use.
Status of immunotherapy in advanced NSCLC: first-line and second-line treatments
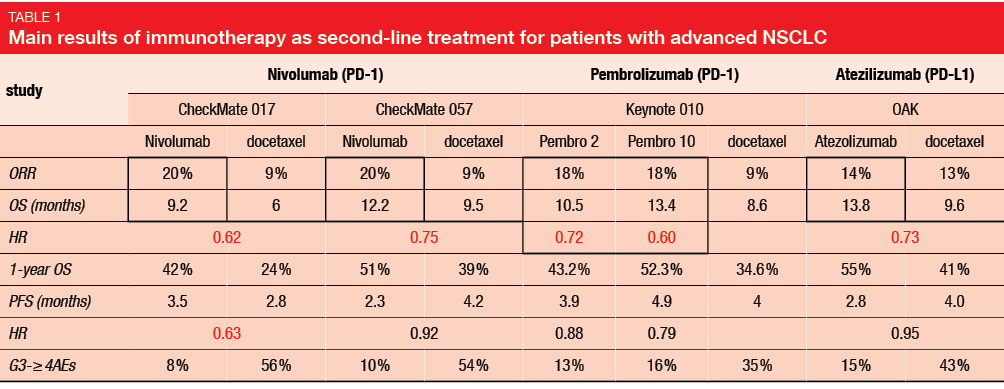
The CheckMate 017 and CheckMate 057 trials compared nivolumab immunotherapy and docetaxel as second-line treatments in advanced NSCLC patients who had failed first-line chemotherapy. In these studies, nivolumab versus docetaxel provided improved objective response rate (ORR; 20 % vs. 9 %) and OS (9.2–12.2 vs. 6.0–9.5 months; CheckMate 017: hazard ratio [HR], 0.62; CheckMate 057: HR, 0.75). Furthermore, the safety results were also more favorable for immunotherapy (grade 3–4 adverse events: 8 %-10 % vs. 54 %-56 %). The subsequent Keynote 010 and OAK studies verified these immunotherapy benefits with comparisons of pembrolizumab and atezolizumab, respectively, to docetaxel for treatment of advanced NSCLC patients, with comparable results (i.e., improved ORR, OS with immunotherapies; Table 1) [1].
Improved efficacy has also been shown for immunotherapy as first-line treatment in advanced NSCLC patients. KEYNOTE-024 compared the single immune agent pembrolizumab to platinum-based chemotherapy as first-line treatment for PD-L1 ≥ 50%, EGFR/ALK-mutant-negative, advanced NSCLC. Here, patients treated with pembrolizumab had improved PFS (10.3 vs. 6 months; HR, 0.50; 95 % confidence interval [CI], 0.37–0.68; p < 0.001), with estimated patient survival at 6 months for pembrolizumab of 80.2 % (95 % CI, 72.9 %–85.7 %), and for chemotherapy of 72.4 % (95 % CI, 64.5 %-78.9 %). Median OS was not reached in either group, although OS was significantly improved with pembrolizumab over chemotherapy (HR for death, 0.60; 95 % CI, 0.41–0.89; p = 0.005) [2].
Immunotherapy combined with chemotherapy has also demonstrated increased patient benefits compared to chemotherapy alone. KEYNOTE-021 cohort G compared pembrolizumab plus chemotherapy with chemotherapy alone as first-line treatment for EGFR/ALK-mutant-negative, advanced NSCLC patients. The combination versus chemotherapy alone provided improved PFS (19.0 vs. 8.9 months; HR, 0.54; 95 % CI, 0.33–0.88; p = 0.0067) and OS (not reached vs. 20.9 months; HR, 0.59; 95 % CI, 0.34–1.05; p = 0.0344) [3]. This pembrolizumab plus chemotherapy PFS of 19 months is particularly encouraging as it is higher than that for the vascular endothelial growth factor inhibitor bevacizumab plus chemotherapy in the ECOG 4599 study (PFS, 12.5 months), which became first-line treatment for advanced NSCLC in the USA [4]. Indeed, at CSCO 2017, Professor Lu from the Lung Tumour Clinical Centre, Shanghai Chest Hospital, Shanghai Jiaotong University (China) indicated that with these data from KEYNOTE-021 cohort G, pembrolizumab plus chemotherapy has already demonstrated superiority over bevacizumab plus chemotherapy. However, the current results are based on a phase II study. Pembrolizumab plus chemotherapy might thus be promoted to first-line treatment for advanced NSCLC instead of bevacizumab plus chemotherapy if the KEYNOTE-189 phase III study provides similar results [5].
Promising results were also obtained in studies that combined different immune agents as first-line treatments for advanced NSCLC patients. CheckMate 012 compared the nivolumab plus ipilimumab combination with nivolumab as single agent, as first-line treatment for stage IIIB or IV NSCLC patients. The ORR was higher for the combination compared to nivolumab alone in the total patient population (43 % vs. 23 %). Furthermore, for the combination therapy, a greatly improved ORR of 92 % was seen for the patients with PD-L1 ≥ 50%. Safety results showed a slightly higher rate of any grade adverse events for the combination compared to nivolumab alone, although the current result remains acceptable [6].
Thus, Professor Lu concluded that anti-PD-L1/PD-1 treatments should be considered as second-line treatments for advanced NSCLC patients. Further studies are still needed to determine whether immunotherapies might become first-line treatments for these patients [5].
Determination of responses to immunotherapy: EGFR-mutation and tumor mutational burden
While the efficacy of immunotherapy has been demonstrated for treatment of advanced NSCLC patients, only a fraction of these patients benefits from anti-
PD-L1/PD-1 therapies. Gainor JF et al. [7] showed that patients with EGFR wild-type or who are heavy smokers are likely to benefit from PD-L1/PD-1 inhibitors, but not patients with EGFR mutations or who are never/ light smokers. Similar results were obtained in the CheckMate 057 and Keynote 010 trials [8].
To determine why the therapeutic efficacies differ between patients with EGFR wild-type and EGFR mutations, Gainor JF et al. [7] analyzed for concurrent PD-L1 expression and CD8+ tumor-infiltrating lymphocytes (TILs). Here, only 4.3 % of the patients showed concurrent high PD-L1 expression (PD-L1+; 50 %) and high levels of CD8+ TILs (TIL+). Teng MW et al. [9] reported that tumor microenvironment can affect the efficacies of anti-PD-L1/PD-1 treatments, whereby patients with type I (TIL+/PD-L1+) tumors were most likely to benefit from single-agent anti-PD-L1/PD-1 therapies. They also suggested that the small proportion of EGFR-mutant NSCLC patients with concurrent TIL+/PD-L1+ might be the reason why poor prognosis with immunotherapy was seen in their full patient group [10].
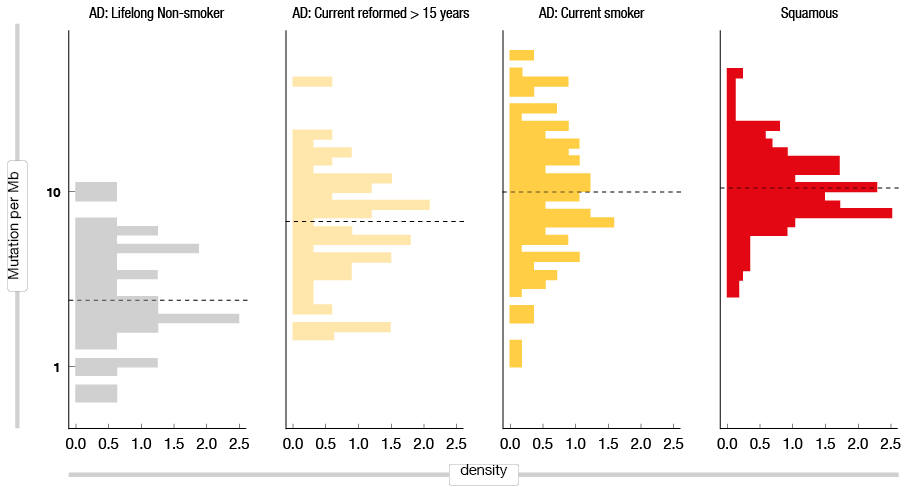
As previously suggested, tumor mutational burden (TMB) might also be a factor in the response to therapy [11]. Gibbons DL et al. [12] compared TMB between current smokers and lifelong nonsmokers, with significantly higher TMB seen for current smokers (Figure 1). Rizvi NA et al. [13] then reported that NSCLC patients with high TMB can obtain longer survival rates than patients with low TMB. Thus, they proposed that the small fraction of TIL+/PD-L1+ patients means that EGFR-mutant tumors are generally not sensitive to immunotherapy. Furthermore, high TMB in the smoker subgroup suggested that smokers are more sensitive to immunotherapy than nonsmokers.
Figure 1: Tumor mutation burden in current smokers compared to life-long non-smokers, for lung adenocarcinomas and squamous cell carcinomas
PD-L1: a good biomarker, or not?
PD-L1 expression has long been used as a prognostic factor or stratification factor in clinical trials. However, more recent evidence suggests that PD-L1 might not be the best biomarker.
CheckMate 012 showed 2-fold to 3-fold greater ORR in patients with PD-L1 ≥ 1 % versus PD-L1 < 1 %. However, KEYNOTE-010 showed that only patients with PD-L1 ≥ 50% have greater OS for pembrolizumab compared to docetaxel. When PD-L1 of 1 % was chosen as the cut-off, no PFS benefit was seen for the PD-L1 ≥ 1 % subgroup with pembrolizumab. Similar results were seen in CheckMate 026, which again showed that patients might not benefit from immunotherapy when PD-L1 of 1 % was chosen as the cut-off. Thus, at CSCO 2017, Professor Fred Hirsch from the University of Colorado, Denver (USA), said that the guidelines for the care of patients with lung cancer indicate 50 % PD-L1 expression as the cut-off. However, these results are from phase II studies, and therefore phase III studies are still needed to confirm them [14].
The further molecular feature of TMB was suggested as a biomarker from the exploratory analysis of CheckMate 026, which stratified the patients into groups who were likely to benefit from immunotherapy versus patients who were not. In the subgroup analysis of CheckMate 026, median PFS for patients with high TMB was significantly greater for nivolumab compared to chemotherapy (9.7 vs. 5.8 months). When the combination of TMB and PD-L1 expression was used as the stratification factor, for the patients treated with nivolumab, those with high TMB and PD-L1 ≥ 50 % showed improved PFS compared to those with low TMB or PD-L1 < 50 %. Professor Hirsch pointed out that TMB might thus represent an alternative biomarker for the selection of NSCLC patients who are likely to benefit from immunotherapy. However, further studies are still needed to combine PD-L1 expression with TMB and/or other biomarkers as stratification determinants to guide clinicians in their selection of the appropriate therapy for NSCLC patients.
Duration of immunotherapy: continuous treatment versus treatment withdrawal
The CheckMate 153 trial compared continuous nivolumab with 1-year fixed-duration nivolumab in patients with advanced NSCLC. Patients in the continuous treatment group showed both greater PFS (not reached vs. 10.3 months) and greater 6-month PFS rates (80 % vs. 69 %). As well as this improved PFS for patients in the continuous treatment group, they also achieved more improved complete/ partial responses (not reached vs. 10.6 months; HR, 0.45; 95 % CI, 0.24–0.85) and stable disease (not reached vs. 9.6 months; HR, 0.44; 95% CI, 0.17–0.19), compared to 1-year fixed-duration nivolumab. Thus, as Professor Lu indicated, continous treatment provides improved results over only 1 year of treatment. However, whether continous treatment will be superior to 2 years of treatment remains to be seen [5].
REFERENCES
- Horn L et al., Nivolumab versus Docetaxel in Previously Treated Patients with Advanced
Non–Small-Cell Lung Cancer: Two-Year Outcomes from Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924-3933. - Reck M et al., Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823-1833.
- Borghaei H et al., Updated Results from KEYNOTE-021 Cohort G: a Randomized, Phase 2 Study of Pemetrexed and Carboplatin (PC) with or without Pembrolizumab (pembro) as First-Line Therapy for Advanced Nonsquamous NSCLC. Annals of Oncology (2017) 28 (suppl_5): v605-v649. 10.1093/annonc/mdx440
- Tyagi P et al., Bevacizumab, When Added to Paclitaxel/Carboplatin, Prolongs Survival in Previously Untreated Patients with Advanced Non–Small-Cell Lung Cancer: Preliminary Results from the ECOG 4599 Trial. Clin Lung Cancer. 2005;6(5):276-278.
- Lu S et al., Progression of Immunotherapy for LC. CSCO 2017
- Hellman MD et al., Nivolumab plus Ipilimumab as First-Line Treatment for Advanced Non–Small-Cell Lung Cancer (CheckMate 012): Results of an Open-Label, Phase 1, Multicohort Study. The Lancet Oncol. 2017;18(1):31-41.
- Gainor JF et al., EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non–Small Cell Lung Cancer: a Retrospective Analysis. Clin Cancer Res. 2016;22(18):4585-4593.
- Lee CK et al., Checkpoint Inhibitors in Metastatic EGFR-Mutated Non–Small Cell Lung Cancer – a Meta-Analysis. J Thorac Oncol. 2017;12(2):403-407.
- Teng MW et al., Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015;75(11):2139-2145.
- Gainor JF et al., The Role of Immunotherapy in Oncogene Driven Lung Cancer. CSCO 2017.
- Alexandrov LB et al., Signatures of Mutational Processes in Human Cancer. Nature. 2013;500(7463):415-421.
- Gibbons DL et al., Smoking, p53 Mutation, and Lung Cancer. Mol Cancer Res. 2014;12(1): 3-13.
- Rizvi NA et al., Cancer Immunology. Mutational Landscape Determines Sensitivity to PD-1 Blockade in Non–Small-Cell Lung Cancer. Science. 2015;348(6230):124-128.
- Hirsch FR et al., PD-L1 vs Tumor Mutation Burden and Other Immune Markers. CSCO 2017.