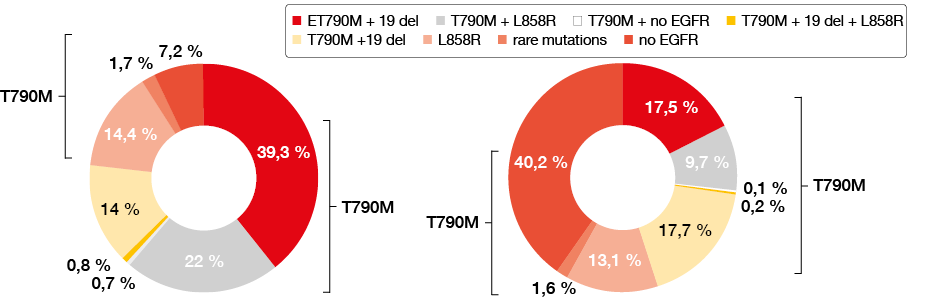Diagnosis of EGFR-mutated NSCLC: from guidelines to reality
Over the last decade, the increasing understanding of critical molecular and cellular mechanisms which drive tumor initiation, maintenance, and progression in non-small-cell lung cancer (NSCLC) have contributed to the discovery of various novel drug targets and the development of new treatment strategies. The standard of care (SOC) for patients with advanced-stage NSCLC is shifting from selecting therapies empirically based on patient clinicopathologic features to using biomarker-driven treatment algorithms according to the molecular profile of the patient’s tumor. The most frequent mutation in Asian patients is EGFR mutation, which occurs in 60.5 % of lung adenocarcinomas (ACs). Thus, to the effective and accurate detection of the EGFR mutation has become important for the selection of subsequent therapies, especially for Asian NSCLC patients. At CSCO 2017, the discussion on the diagnosis of EGFR-mutated NSCLC mainly focused on how to choose the appropriate method for detecting EGFR mutation. Another topic of intense discussion was recent progress in the field of mechanisms of resistance to third-generation EGFR TKIs.
Suitable shoes for appropriate feet – ARMS, IHC, NGS, tissue biopsy or liquid biospy?
Molecular subtyping of LC is essential for selecting the appropriate therapeutic strategy. Precision oncology is now the evidence-based SOC for the management of many patients with advanced NSCLC. The application of palliative targeted therapies consisting of oral TKIs such as gefitinib, erlotinib and afatinib in advanced/metastatic lung ACs harboring EGFR abnormalities has consistently contributed to more favorable outcomes compared to the use of traditional cytotoxic agents. However, choosing a suitable method for the accurate, rapid and consistent detection of EGFR mutations or other mutations, is gaining importance in the treatment of NSCLC. Chinese guidelines for the treatment of NSCLC, developed based on expert consensus, define minimum requirements for routine testing and optional strategies for the identification of EGFR mutations in advanced NSCLC [1].
Among methods frequently used to detect EGFR mutation, Sanger Sequencing is the most sensitive procedure, followed by Amplification Refractory Mutation System (ARMS) and Next-Generation Sequencing (NGS). According to the 2017 CSCO guidelines for the detection of molecular subtypes of advanced non-squamous NSCLC, ARMS should be used as the standard strategy for the detection of EGFR mutation in patients who were diagnosed by immunohistochemistry (IHC). Only when tissue is difficult to obtain, a blood sample is the optional choice for ARMS detection. However, the recommendations differ from the National Comprehensive Cancer Network (NCCN) guideline for ACs, which recommends subtyping by NGS to detect EGFR and ALK mutations. One explanation for this discrepancy might be the imbalance of health resources across different regions, and the value of diagnosis.
With regard to osimertinib, a third-generation EGFR TKI that was approved by the China Food and Drug Administration as second-line treatment of EGFR-mutant advanced NSCLC, the Chinese guidelines recommend that the T790M mutation should be detected by tissue biopsy using PCR-ARMS, Cobas, NGS or ddPCR. Only when tissue samples are difficult to acquire, liquid biopsy by PCR-ARMS, super-ARMS, Cobas, cDNA-NGS or ddPCR should be considered as a standard strategy in advanced non-squamous NSCLC patients who were resistant to first-line EGFR TKI treatment.
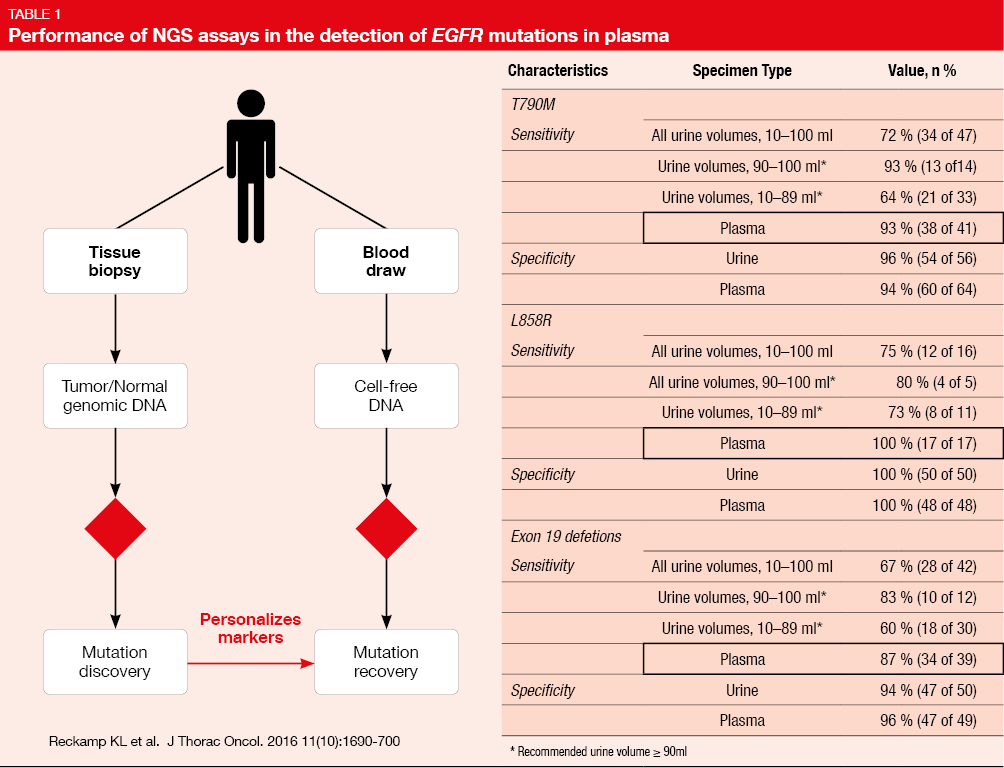
Liquid biopsies are based on the detection of DNA fragments in blood, sweat, urine and other liquids obtained from human beings by high-throughput DNA sequencing such as NGS. The main subjects of liquid biopsies are circulating tumor DNA (ctDNA), circulating tumor cells (CTC) and exosomes. As reported by Reckamp KL et al. [2], the sensitivity for the detection of EGFR T790M and L858R mutations as well as deletion 19 in plasma was 93 %, 100%, and 87 %, respectively, and the specificity was 94 %, 100%, and 96 %, respectively (Table 1).This indicated a suitable performance of mutation enrichment NGS assays for the detection of EGFR mutations in advanced NSCLC. Meanwhile, Thress KS et al. also reported that EGFR T790M mutation and EGFR C797S mutation can be detected by ctDNA liquid biopsy in patients resistant to EGFR TKI treatment [3]. Sundaresan et al. indicated high consistency of liquid biopsy by CTC, ctDNA, CTC/ctDNA with tissue biopsy (74 %, 62 % and 69 %) in relation to the detection of T790M mutation [4].
Thus, the 2017 CSCO guideline recommends that tissue biopsy by ARMS should be the standard strategy for molecular subtyping of advanced NSCLC patients who were diagnosed by IHC. Even though liquid biopsy displays higher sensitivity and specificity in detecting EGFR mutations or EGFR T790M after resistance to EGFR TKI, the 2017 CSCO guideline recommended liquid biopsy as an optional choice only when tissue biopsy was not available. In summary, CSCO developed the guidelines for the diagnosis of Chinese NSCLC patients to consider several aspects and not only the sensitivity and specificity of the respective technology.
New insights on resistance mechanisms of osimertinib – focus on the L792 mutation
Osimertinib, a third-generation EGFR TKI that selectively inhibits both EGFR TKI–sensitizing and EGFR T790M resistance mutations, has changed the second-line SOC for EGFR-mutant advanced NSCLC. The phase III FLAURA study focused on the efficacy of osimertinib in previously untreated, EGFR-mutant (exon 19 deletion or L858R mutation) patients with advanced NSCLC. The results of FLAURA showed higher PFS for osimertinib compared to first-generation EGFR TKIs. However, acquired resistance was again inevitable. EGFR C797S mutation was suggested to represent the most notable resistance mechanism to this drug, but other EGFR mutations may also exist.
At CSCO 2017, professor Caicun Zhou from the Tongji University Medical School presented a case report, where a novel mutation of EGFR at Leu792 was reported which may represent another resistance mechanism to osimertinib [6]. Patients with stage IV lung ACs, who progressed on the third-generation TKI osimertinib when administered as second-line treatment, were investigated. Plasma samples and samples obtained from pleural effusions were collected for cell free DNA (cfDNA) and sequenced by NGS for 416 cancer-related genes. The results showed that besides the most common T790M/C797S mutations, Leu792 was also mutated, including the mutations L792F, L792Y and L792H. In-depth analyses and structural predictions suggest a role of C797S mutation in the interruption of osimertinib binding to EGFR [7].
Rate of T790M mutation – data from studies in China
The first- and second-generation EGFR TKIs can significantly prolong PFS in patients with advanced, EGFR-mutant NSCLC. However, resistance has been observed. EGFR T790M mutation occurs in 60 % of patients resistant to first- and second-generation EGFR TKIs [8]. While the data on T790M has mainly been obtained from foreign studies, at CSCO 2017, a large sample study was presented that evaluated the T790M mutation rate specifically for Chinese patients [9].
The study was conducted in 79 centers across 32 cities in the northern, eastern, middle and southern areas of China. A total of 2,693 samples, including 1,427 blood samples (53 %) and 1,266 tissue samples (47 %), were obtained from patients who developed progressive disease (PD) after EGFR TKI treatment since March 31st, 2017. Among all tissue samples, 81.7 % were obtained from tissue biopsies. In 91.8 % of the samples, the percentage of cancer cells was more than 10 %. T790M mutations were detected in 62.8 % and 27.5 % by tissue and blood examination, respectively (Figure 1). Results according to tissue examination showed that in T790M-mutant patients, 39.3 %, 22 %, 0.7 % and 0.8 % of patients simultaneously had 19 deletion, L858R mutation, rare mutations (or both exon 19 deletion and L858R mutation) and no other EGFR mutations, respectively. Patients without any EGFR mutations were only observed in 7.2 % of cases. Results by blood examination showed that in EGFR T790M-mutant patients, 17.5 %, 9.7 %, 0.2 % and 0.1 % had 19 deletion, L858R mutation, rare mutations and no other EGFR mutations, respectively. Patients without any EGFR mutations were observed in 40.2 % ofcases. Subgroup analysis showed no differences with regard to age, sex, amount, method, or site of the tissue obtained. The mutation ratio of T790M was 50 % and 63.9 % in tissues with fractions of tumor cells of < 10 % and ≥10 %, respectively. This means that even in biopsies with a fraction of tumor cells below 10 %, T790M mutation detection could be used as a marker for diagnosis.
Thus, Professor Ying Cheng from the Cancer Hospital of Jilin Province, Changchun (China), indicated that the prevalance of the T790M mutation was 62.8 % by tissue examination, which is similar to results from previous studies. Relatively low sensitivity for the detection of T790M was found in blood examinations, which suggests that more sensitive methods will be needed in the future [8].
Figure 1: The rate of T790M rate according to tissue (left) or blood (right) examination
REFERENCES
- Li Y et al., Molecular typing for LC CSCO 2017
- Reckamp KL et al., A highly sensitive and quantitative test platform for detection of NSCLC EGFR mutations in urine and plasma. J Thorac Oncol. 2016 Oct; 11(10):1690-700.
- Thress KS et al., Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med. 2015 Jun; 21(6):560-2.
- Sundaresan TK et al., Detection of T790M, the acquired resistance EGFR mutation, by tumor biopsy versus noninvasive blood-based analyses. Clin Cancer Res. 2016 Mar 1 ;22(5):1103-10.
- Soria JC et al., Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N Engl J Med. 2018 Jan 11;378(2):113-125. doi: 10.1056/NEJMoa1713137.
- Caicun Zhou et al., Development of precision medicine in advanced NSCLC CSCO 2017
- Chen K et al., Novel mutations on EGFR Leu792 potentially correlate to acquired resistance to osimertinib in advanced NSCLC. J Thorac Oncol. 2017 Jun;12(6):e65-e68.
- Camidge DR et al., Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol. 2014 Aug;11(8):473-81.
- Cheng Y et al., T790M rate in Chinese advanced NSCLC after resistance to EGFR TKI CSCO 2017