EGFR-mutated disease: early combinations and new approaches in exon 20 insertion-positive lung cancer
Upfront radiation plus TKI in the oligometastatic setting
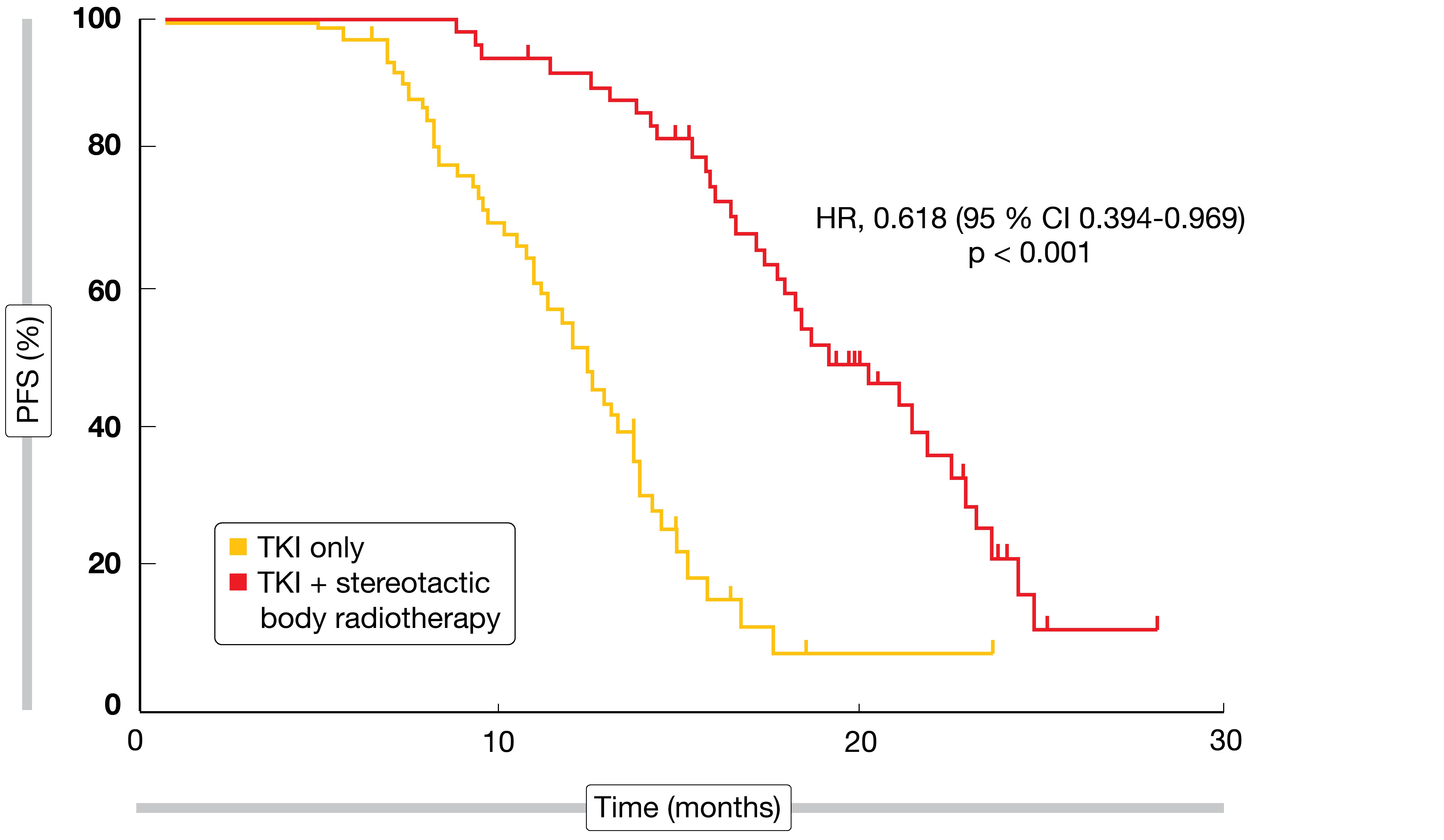
Oligometastatic disease is generally defined by one to five metastatic lesions. As progression occurs most frequently in sites of the original disease, it is surmised that aggressive local treatment might prevent further dissemination. Based on this rationale, the open-label, randomized, phase III SINDAS trial conducted in China explored the use of concurrent stereotactic body radiotherapy (SBRT) and EGFR TKI therapy in patients with oligometastatic, EGFR-mutant NSCLC [1]. Patients had no more than two metastatic lesions in any one organ and a maximum of five metastases in total. In the experimental arm (n = 68), SBRT was administered at doses of 25 to 40 Gy in five fractions, while patients in the control arm (n = 65) received TKI treatment (i.e., gefitinib, erlotinib, icotinib) only.
PFS was defined as the primary endpoint. Here, the combined regimen gave rise to a significant benefit with a 38 % reduction in the risk of progression or death (HR, 0.618; p < 0.001; Figure 1). Also, the addition of radiotherapy prolonged OS in a significant manner (HR, 0.682; p < 0.001). At the same time, the incidence of grade-3 AEs including rash, severe liver injury, pneumonitis, esophagitis, and pathological rib fractures did not differ significantly across the treatment arms. Overall, these findings confirmed previous hypotheses assuming a benefit of consolidative SBRT for limited metastatic NSCLC. The authors concluded that aggressive upfront local therapy should be investigated further in large phase III trials as a standard option in this clinical scenario.
First-line dual TKI therapy: osimertinib plus gefitinib
As is known, the first-generation EGFR TKI gefitinib and the third-generation EGFR TKI osimertinib give rise to different predominant second-site EGFR resistance mutations (i.e., T790M and C797S with gefitinib and osimertinib, respectively). Each agent retains activity against the main resistance mechanism observed with the other one. Based on the assumption that treatment response might be prolonged with a combined strategy, a phase I/II study is currently evaluating the first-line regimen of gefitinib plus osimertinib in patients with EGFR-mutant (i.e., EGFR L858R mutation or exon 19 deletion), stage IV NSCLC.
Twenty-seven patients were included in the analysis presented at the ASCO Congress [2]. Most of these were Caucasian (81 %), and more than half had never smoked. Treated or asymptomatic untreated CNS disease was permitted; 33 % and 26 % of patients had treated and untreated brain lesions, respectively, while in 41 %, CNS metastases were absent. Primary endpoints included the maximum tolerated dose and the feasibility of treatment, which was defined as receipt of the combination therapy for at least six 28-day cycles. No dose-limiting toxicities occurred with gefitinib 250 mg and osimertinib 80 mg daily during the dose escalation phase, and AEs were consistent with the known toxicity profile for EGFR TKI therapy. Rash (96 %), diarrhea (85 %) and dry skin (70 %) represented the most common events. None of the patients experienced pneumonitis.
During the dose expansion phase, the feasibility endpoint was met by 81.5 % of patients who received at least six cycles. Objective responses and disease control resulted in 88.9 % and 100 %, respectively. Median PFS was 22.5 months, although these data are still immature, as are those for OS. Moreover, the combination induced rapid and near-universal plasma clearance of the mutant EGFR allele, with 88 % of patients showing undetectable mutation status at two weeks. In terms of acquired resistance, next-generation sequencing at disease progression revealed no known pathogenic EGFR second-site mutations in seven patients. Also, no patient experienced histologic transformation.
In their summary, the investigators pointed out that the observed ORR of 88.9 % is comparable to response rates obtained for first-line use of osimertinib. Further analyses of PFS and OS will facilitate understanding of the clinical utility of first-line dual EGFR TKI therapy.
Mobocertinib in NSCLC with EGFR exon 20 insertions
Among activating EGFR mutations, exon 20 insertions mark a type of NSCLC that is difficult to treat and associated with poor prognosis. These tumors are generally insensitive to EGFR TKI therapy, and treatment options are limited after progression on platinum-based chemotherapy.
The novel EGFR TKI mobocertinib (TAK-788) is currently in development for lung cancer with exon 20 insertions and has already received Breakthrough Therapy Designation by the US Food and Drug Administration for the treatment of patients with metastatic NSCLC harboring EGFR exon 20 insertions whose disease has progressed on or after platinum-based chemotherapy.
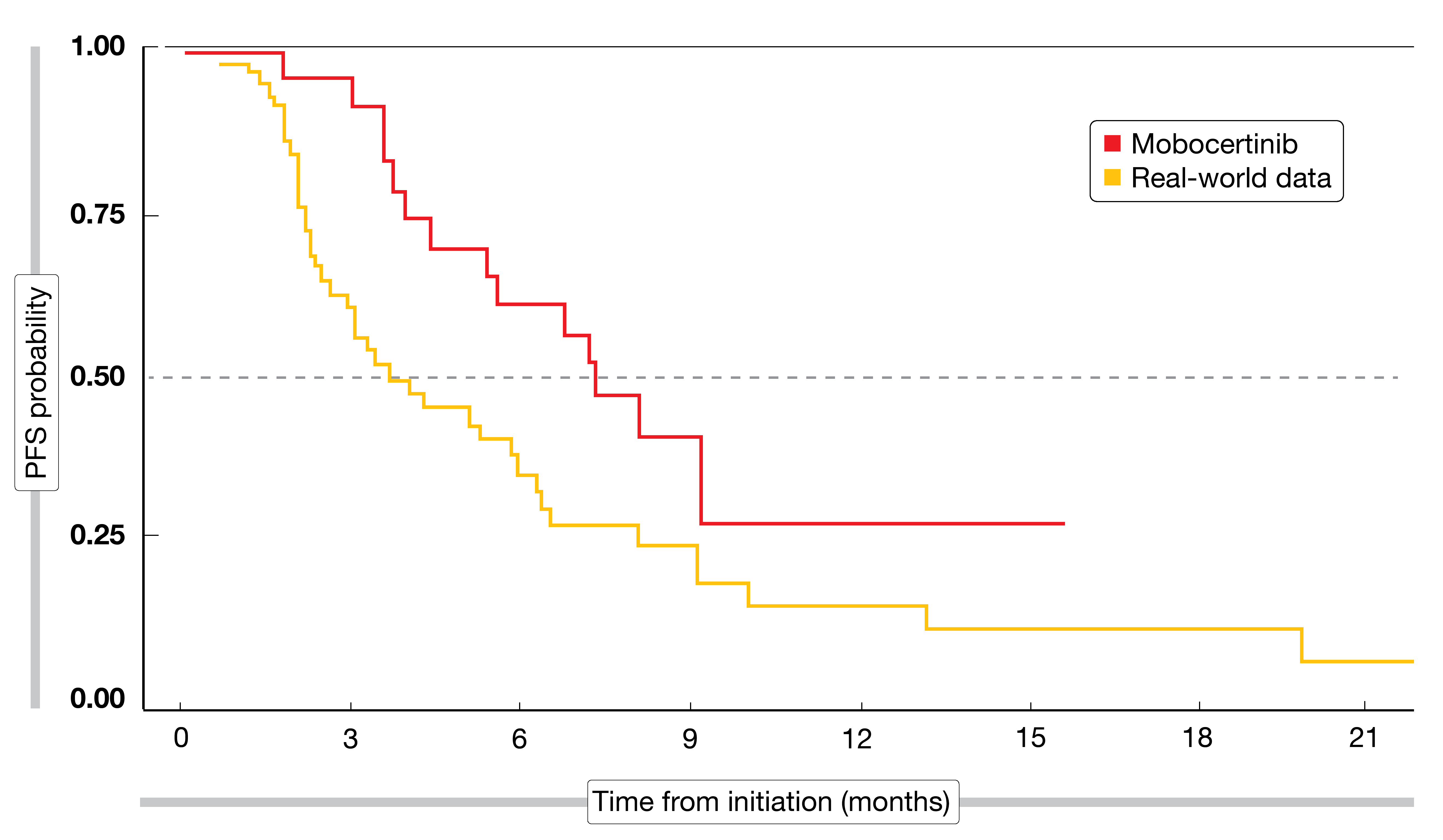
In the absence of head-to-head evidence, Horn et al. indirectly compared clinical trial data for mobocertinib obtained in a single-arm, phase I/II study with real-world outcomes [3]. Real-world data that had been generated to understand the natural history and treatment patterns in patients with exon 20 insertions were obtained from the US Flatiron Health HER-derived de-identified database. In the ongoing phase I/II trial, mobocertinib is being administered orally at a daily dose of 160 mg. A total of 99 patients with locally advanced or metastatic NSCLC harboring EGFR exon 20 insertions (n = 28 and n = 71 for mobocertinib and real-world patients, respectively) were included in the analysis; data were reported for the second-line setting. Treatments in the real-world population included chemotherapy, immunotherapy, EGFR TKI treatment and combinations of these; also, combinations of chemotherapy and/or EGFR TKIs with monoclonal antibodies were used. Immunotherapies were most prevalent, at 29.6 %, followed by EGFR TKI treatment (25.4 %) and docetaxel (10.0 %).
Even with baseline propensity matching, mobocertinib performed better than the comparator regimens. Patients in the mobocertinib group achieved superior ORR (43 % vs. 14 %; p = 0.003) and PFS (7.3 vs. 3.7 months; p = 0.0235; Figure 2). A trial comparing first-line mobocertinib with platinum-based chemotherapy in NSCLC patients with EGFR exon 20 insertions is currently recruiting (NCT04129502).
Poziotinib: Cohort 1 of the ZENITH20 study
Similarly, the oral, irreversible EGFR TKI poziotinib has been developed to target EGFR and HER2 exon 20 insertions. At the ASCO Congress, Le et al. presented the results for Cohort 1 of the multicenter, phase II ZENITH20 trial that assessed poziotinib in a total of seven cohorts including previously treated and treatment-naïve NSCLC patients [4]. Brain metastases were permitted if the lesions were stable.
Cohort 1 contained 88 evaluable patients with EGFR exon 20 insertions who received poziotinib after pretreatment. In this group, the TKI gave rise to an ORR of 19.3 % and DCR of 80.7 %. Duration of response was 7.4 months. Assessment of response according to prior therapy showed that after ≥ 3 lines of treatment, patients obtained even slightly better ORR (22.2 %) than after one line (18.9 %) or two lines (16.7 %). The investigators concluded that multiple prior lines of therapy did not impair response. EGFR insertion location had a certain effect on the efficacy of treatment: exon 20 near-loop insertions were the most prevalent alterations (> 50 %), and these patients benefited most from poziotinib.
Tumor shrinkage occurred in 84 % of evaluable patients. Freedom from progression was maintained for a median of 4.1 months. Twelve patients had stable CNS disease at baseline. Among these, 83 % did not experience progression during treatment, and only 3 % of patients without baseline brain lesions developed new CNS metastases. Common grade-3 treatment-related AEs events comprised diarrhea (25 %), rash (28 %), stomatitis (9 %), and paronychia (6 %).
EGFR-MET–bispecific antibody amivantamab
A novel treatment approach with broad application in EGFR-mutant NSCLC is the EGFR-MET–bispecific antibody amivantamab that targets both activating and resistance EGFR mutations and MET mutations/amplifications. This agent inhibits aberrant EGFR and MET signaling through binding to the extracellular domains of these receptors, rather than targeting the kinase active site.
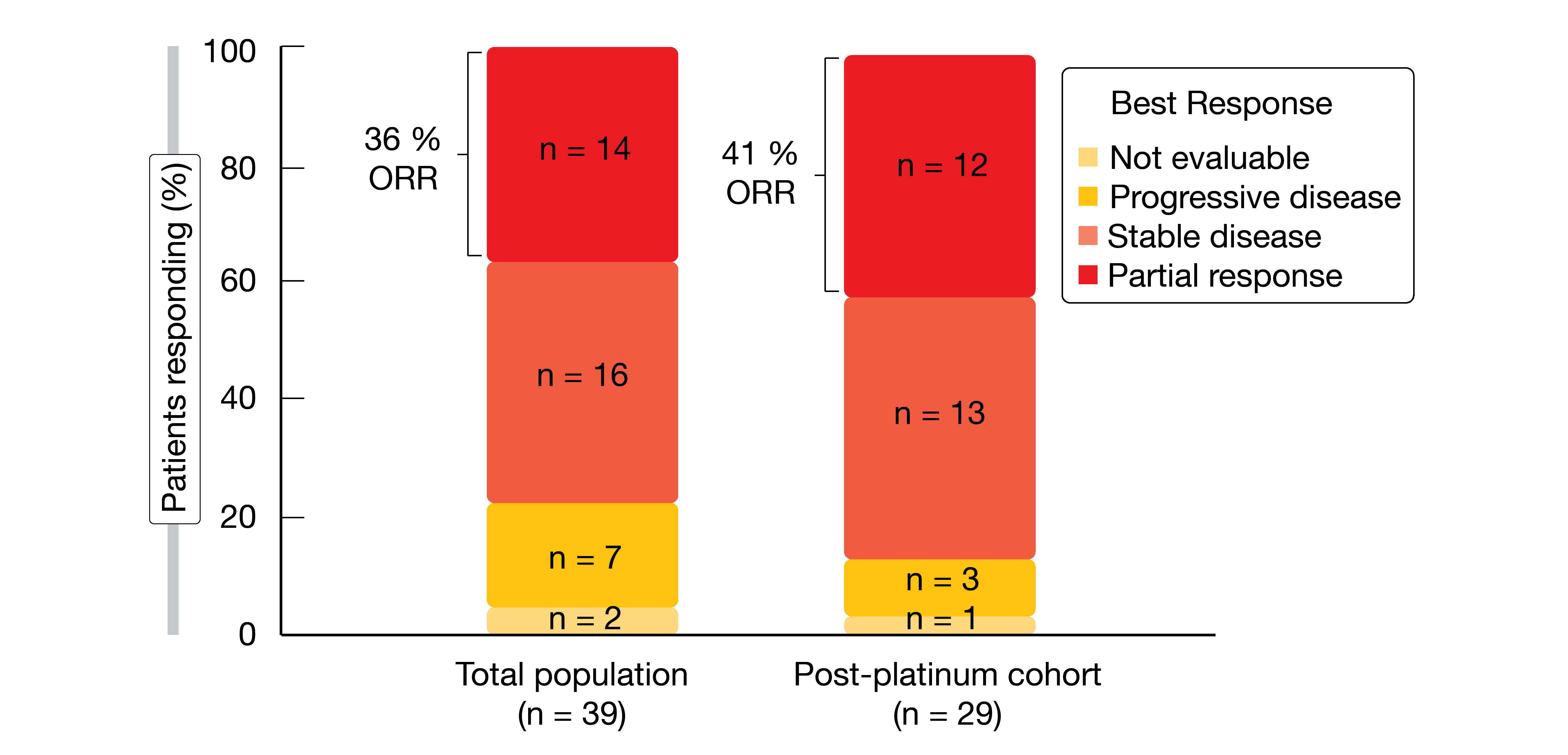
Amivantamab is being investigated in the ongoing phase I CHRYSALIS trial in patients with metastatic or unresectable NSCLC and activating EGFR or MET mutations or amplifications. Park et al. reported preliminary results for patients with EGFR exon 20 insertions who had received the recommended phase II dose of 1,050 mg (1,400 mg for patients weighing ≥ 80 kg) intravenously once weekly for the first cycle and biweekly thereafter [5]. Fifty and 39 patients constituted the safety and response-evaluable populations, respectively. In the response-evaluable group, 29 (74 %) had been treated with platinum-based chemotherapy in the metastatic setting prior to inclusion, while six were treatment-naïve and four had received other therapies including EGFR TKIs and/or VEGF inhibition.
Responses were observed in both treatment-naïve and post-platinum patients. ORRs amounted to 36 % and 41 % in the overall group and in the post-platinum cohort, respectively (Figure 3). In the total population, 67 % of patients derived clinical benefit; for the post-platinum group, this was 72 %. Activity of treatment was observed across all 13 distinct EGFR exon 20 insertion alterations identified. Responses were durable, with a median duration of 10 months for all evaluable patients and 7 months for the post-platinum group. Median PFS was 8.3 and 8.6 months, respectively.
Amivantamab was shown to have a manageable safety profile, with rash, infusion-related reaction and paronychia occurring as the most common all-grade events. Toxicities were mostly grade 1 and 2. Dose reductions and discontinuations due to AEs were infrequent, at 10 % and 6 %. Based on these data, amivantamab has received FDA Breakthrough Therapy Designation for the treatment of patients with EGFR exon 20 insertion-mutant NSCLC whose disease has progressed on or after platinum-based chemotherapy.
High-dose osimertinib as another option
The activity of third-generation EGFR TKIs such as osimertinib in NSCLC with EGFR exon 20 insertions is unknown. Preclinical studies suggest that the favorable therapeutic window of these agents might allow for inhibition at clinically achievable doses [6]. Therefore, the single-arm, phase II EA5162 trial assessed osimertinib 160 mg in 17 patients with advanced NSCLC harboring EGFR exon 20 insertions who had received at least one prior treatment line [7]. Notably, the dose used in this trial was double the approved osimertinib dose.
Osimertinib 160 mg daily showed clinical activity in exon 20 insertion-mutant NSCLC, with a confirmed ORR of 24 %. Eighty-two percent of patients achieved disease control. Median duration of response had not been reached yet, and median PFS was 9.6 months. AEs were consistent with previous reports. Diarrhea, fatigue, cytopenia and anorexia occurred as the most common toxicities, with low rates of grade ≥ 3 events. Skin toxicities were restricted to grade 1 AEs. One patient experienced grade 4 respiratory failure, and another discontinued study treatment due to grade 3 anemia. Further study of osimertinib in patients with EGFR exon 20 insertions is planned.
REFERENCES
- Wang XS et al., First-line tyrosine kinase inhibitor with or without aggressive upfront local radiation therapy in patients with EGFRm oligometastatic non-small-cell lung cancer: interim results of a randomized phase III, open-label clinical trial (SINDAS). J Clin Oncol 38: 2020 (suppl; abstr 9508)
- Rotow JK et al., Concurrent osimertinib plus gefitinib for first-line treatment of EGFR-mutated non-small cell lung cancer (NSCLC). J Clin Oncol 38: 2020 (suppl; abstr 9507)
- Horn L et al., Indirect comparison of mobocertinib (TAK-788) vs real-world data outcomes in refractory NSCLC with EGFR exon 20 insertions. J Clin Oncol 38: 2020 (suppl; abstr 9580
- Le X et al., Poziotinib shows activity and durability of responses in subgroups of previously treated EGFR exon 20 NSCLC patients. J Clin Oncol 38: 2020 (suppl; abstr 9514)
- Park K et al., Amivantamab (JNJ-61186372), an anti-EGFR-MET bispecific antibody, in patients with EGFR exon 20 insertion (Exon20ins)-mutated non-small cell lung cancer (NSCLC). J Clin Oncol 38: 2020 (suppl; abstr 9512)
- Hirano T et al., In vitro modeling to determine mutation specificity of EGFR tyrosine kinase inhibitors against clinically relevant EGFR mutants in non-small-cell lung cancer. Oncotarget 2015; 6(36): 38789-803
- Piotrowska Z et al., ECOG-ACRIN EA5162: A phase II study of high-dose osimertinib in NSCLC with EGFR exon 20 insertions. J Clin Oncol 38: 2020 (suppl; abstr 9513)