BTK inhibition in Waldenström’s macroglobulinemia: trial updates and real-world insights
The open-label, multicenter, randomized phase III ASPEN trial was set up to assess the efficacy and safety of the potent, selective, irreversible next-generation BTK inhibitor zanubrutinib in Waldenström’s macroglobulinemia (WM). Cohort 1 of the study included patients with MYD88-mutated disease (n = 201); here, zanubrutinib was compared to ibrutinib after 1:1 randomization. In Cohort 2, 28 patients with MYD88 wildtype received zanubrutinib in a non-randomized manner. Both treatment-naïve and pretreated patients participated, although no prior BTK inhibition was allowed. Untreated patients were eligible when considered unsuitable for standard chemoimmunotherapy.
With respect to the primary endpoint, which was complete response (CR) plus very good partial response (VGPR) for zanubrutinib vs. ibrutinib, no significant difference resulted at the time of the primary analysis [1], although the findings indicated clinically meaningful efficacy and improved tolerability of the newer BTK inhibitor.
42-month follow-up of ASPEN
This impression was corroborated by the long-term follow-up reported at EHA 2022 [2]. In Cohort 1, the CR plus VGPR rate was numerically higher at all time points with zanubrutinib vs. ibrutinib. At 44.1 months, this was 36.3 % vs. 25.3 %, with shorter median time to CR plus VGPR (6.7 vs. 16.6 months). Zanubrutinib elicited a higher event-free rate for the duration of CR plus VGPR at 24 months (90.6 % vs. 79.3 %). Median PFS and OS had not yet been reached, with hazard ratios favoring zanubrutinib (PFS, 0.63; OS, 0.75). At 42 months, 87.5 % vs. 85.2 % of patients were alive, and 78.3 % vs. 69.7 % were progression-free. After almost 4 years, 66 % vs. 52 % remained on treatment.
Assessments by CXCR4 status demonstrated that patients with CXCR4 mutation derived benefits from zanubrutinib compared to ibrutinib regarding VGPR or better (21.2 % vs. 10.0 %), major response (78.8 % vs. 65.0 %), time to major response (3.4 vs. 6.6 months), and PFS (HR, 0.50). Seventy-three percent of these patients treated with zanubrutinib enjoyed freedom from progression at 42 months, while only 49.0 % in the ibrutinib arm did.
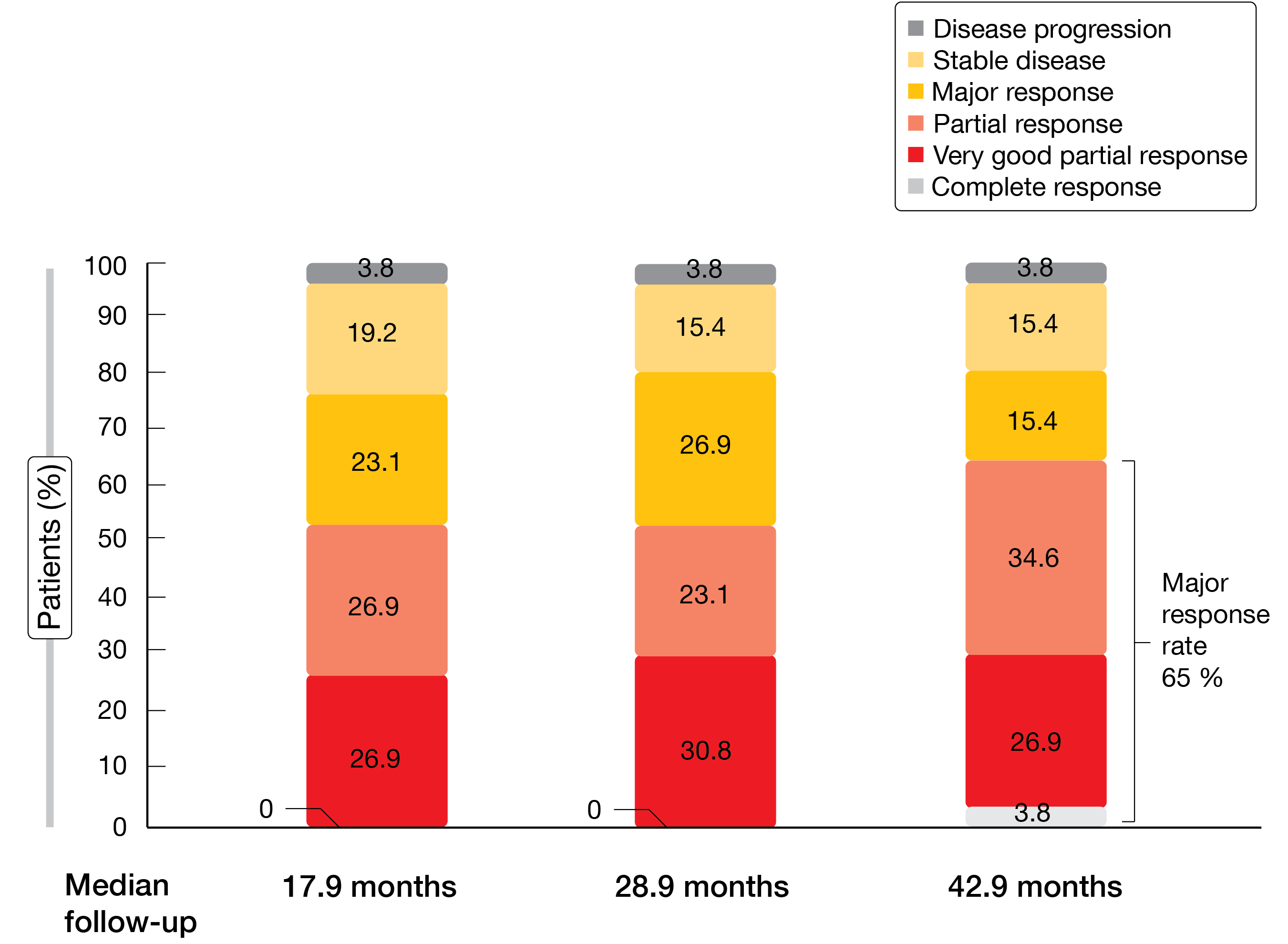
In Cohort 2, responses to zanubrutinib treatment continued to deepen over time (Figure). At 42.9 months of follow-up, one patient had achieved complete response, and the rate of major responses had risen to 65 %. Event-free rates of PFS and OS at 42 months were 53.8 % and 83.9 %, respectively.
Likewise, the safety advantages of zanubrutinib remained consistent over time, with less off-target activity versus ibrutinib. Zanubrutinib-treated patients showed lower cumulative incidences of atrial fibrillation, diarrhea, hypertension, muscle spasms, and pneumonia, and had fewer AEs leading to death, treatment discontinuation, or dose reductions. Among AEs of special interest observed in cohort 1, neutropenia was more common with zanubrutinib (all grades, 34.7 % vs. 20.4 %), although infection rates were similar across the arms (all grades, 79.2 % vs. 79.6 %), with grade 3/4 events occurring less frequently in the experimental arm (21.8 % vs. 27.6 %). The authors noted that over time, zanubrutinib showed a consistent trend of deeper, earlier, and more durable responses compared with ibrutinib.
Figure: ASPEN trial: deepening of responses to zanubrutinib treatment over time
Expanded access study of zanubrutinib
A phase II, expanded access study was initiated at 10 academic and community medical centers across the United States to provide real-world experience with zanubrutinib in patients with WM for whom no other clinical trials were available. Castillo et al. presented results for 17 treatment-naïve and 33 pretreated patients at EHA 2022 [3]. Forty-one were assigned to receive zanubrutinib 160 mg BID, while 9 received zanubrutinib 320 mg QD. Compared to the population recruited into the ASPEN trial, the patients were older on average, had worse ECOG performance status, longer disease duration, and poorer prognosis. Most of them had either intermediate-risk or high-risk disease (54.0 % and 40.0 %, respectively). In the group with relapsed/refractory disease, the median number of prior therapies was 2.
Nevertheless, the response rates and toxicity profile were comparable to the findings obtained in the ASPEN study. Overall, 85.4 % of patients responded to treatment, with 73.2 % achieving a major response and 39.0 % obtaining VGPR. Responses were similar across the treatment lines, as well as in patients receiving 160 mg BID and 320 mg QD. The ORR was lower than that observed in ASPEN, which had bordered on 100 %. According to the authors, this difference might be attributed to less frequent response assessments (every 6 months), as any responses arising in between would not have been captured. For instance, 3 of 4 patients with disease progression as best response had IgM levels indicating improvement during the first 6 months, i.e., prior to the first response assessment. Median PFS and OS had not been reached yet at the time of the analysis.
No new safety signals emerged during the study period. Overall, 6.0 % and 8.0 % of treatment-emergent AEs led to treatment discontinuation and dose reductions, respectively; dose interruptions became necessary in 12.0 %. No fatal TEAEs were reported. The safety profile did not differ in any meaningful way between patients with untreated or relapsed/refractory disease and those assigned to 160 mg BID or 320 QD. The authors concluded that these findings were consistent with the established zanubrutinib profile in WM and other B-cell malignancies when administered as monotherapy at a daily dose of 320 mg orally in patients with intermediate-risk or high-risk, treatment-naïve or relapsed/refractory WM.
Long-term findings for single-agent acalabrutinib
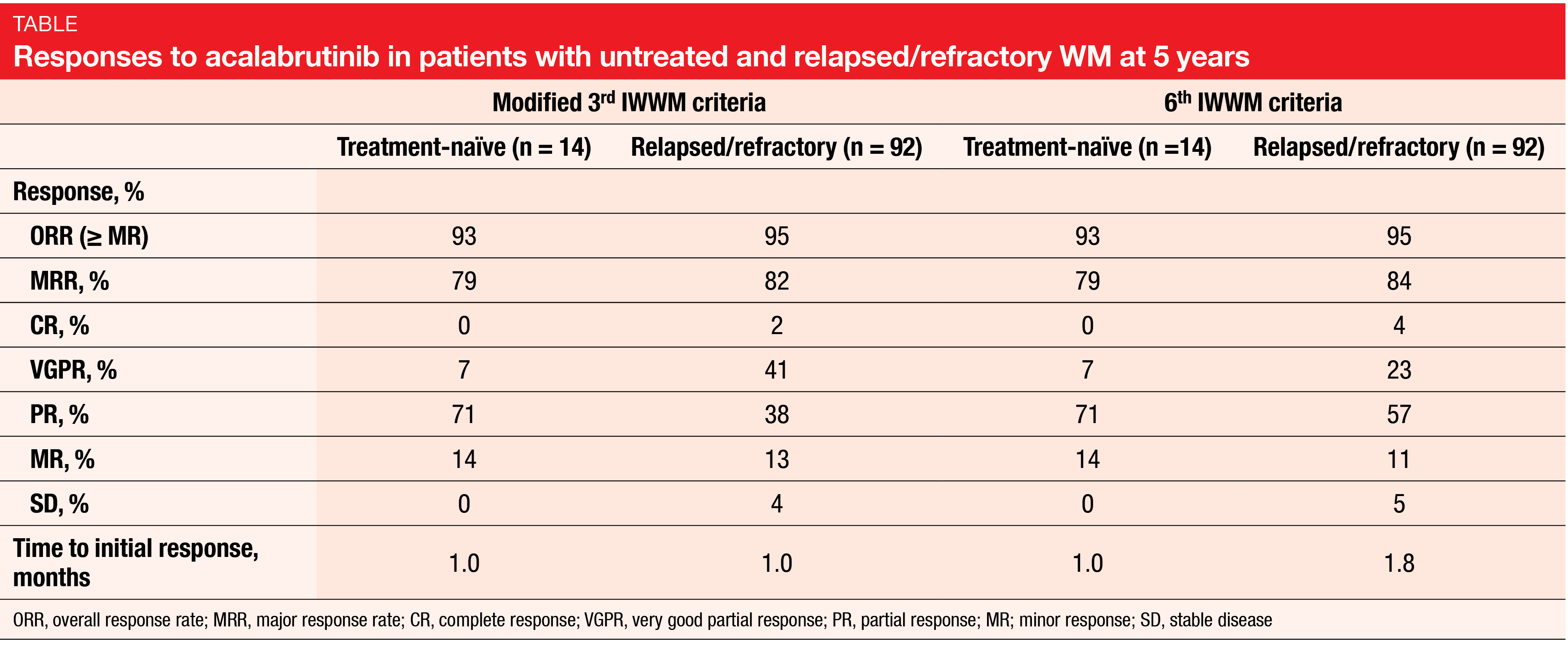
In the phase II ACE-WM-001 study, monotherapy with the highly selective, potent, covalent BTK inhibitor acalabrutinib has shown durable responses and a tolerable safety profile in patients with treatment-naïve (n = 14) and relapsed/refractory (n = 92) WM [4]. Acalabrutinib was administered at doses of 100 mg BID or 200 mg QD until disease progression. Owen et al. reported the final results of the trial after a median follow-up of 63.7 months [5]. Approximately half of patients remained on treatment at the time of data cutoff.
Acalabrutinib therapy induced ORRs of 93 % and 95 % in patients with untreated and relapsed/refractory WM, respectively (Table). Responses were consistent irrespective of age, baseline ECOG performance status, baseline hemoglobin and IgM levels, and prior treatment lines. The PFS and OS benefits were maintained over time in both cohorts. At 66 months, PFS rates were 84 % and 52 % for treatment-naïve and relapsed/refractory patients, respectively; OS rates amounted to 91 % and 71 %, respectively.
Acalabrutinib exhibited a tolerable safety profile with no new toxicities and a low incidence of individual cardiovascular events. The most common grade 3/4 AEs were neutropenia (17 %), pneumonia (9 %), anemia (6 %), and hyponatremia (5 %). Key events of clinical interest included cardiac events (all grades, 21 %; grade 3/4, 8 %), bleeding (62 %; 6 %), and hypertension (7 %; 4 %). Atrial fibrillation and hypertension showed low cumulative incidences over time, while infections and bleeding events slightly increased, although these were mostly low-grade. AEs led to treatment discontinuation in 29 % and 17 % of untreated and pretreated patients, respectively. Overall, acalabrutinib appeared to be a highly effective treatment that provides durable responses with a tolerable safety profile in patients with treatment-naïve or relapsed/refractory WM.
REFERENCES
- Tam CS et al., A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinema: the ASPEN study. Blood 2020; 136(18): 2038-2050
- Dimopoulos M et al., ASPEN: long-term follow-up results of a phase 3 randomized trial of zanubrutinib versus ibrutinib in patients with Waldenström macroglobulinemia. EHA 2022, P1161
- Castillo JJ et al., Results of a phase 2 expanded access study of zanubrutinib in patients with Waldenström macroglobulinemia. EHA 2022, P1160
- Byrd JC et al., Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol 2021; 39(31): 3441-3452
- Owen R et al., Acalabrutinib in treatment-naïve or relapsed/refractory Waldenström macroglobulinemia: 5-year follow-up of a phase 2, single-arm study. EHA 2022, P1130
© 2022 Springer-Verlag GmbH, Impressum