Reducing risks further in chronic lymphocytic leukemia
Treatment-naïve CLL
SEQUOIA arm D: zanubrutinib in addition to venetoclax
The second-generation BTK inhibitor zanubrutinib is being tested in the phase III SEQUOIA trial in the setting of untreated chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) with and without del(17p). Zanubrutinib monotherapy has shown high tolerability and efficacy in arm C of the study that included patients with del(17p) [1]. At EHA 2024, Ma et al. reported preliminary results for 66 individuals harboring del(17p) and/or TP53 mutation who received zanubrutinib plus venetoclax in arm D [2]. The combination was started in cycle 4 following a zanubrutinib lead-in and was continued for 12-24 cycles.
Venetoclax and zanubrutinib could be discontinued early after ≥12 and ≥ 27 cycles, respectively, if complete response (CR) or CR with incomplete hematological recovery (CRi) confirmed by bone marrow biopsy was achieved together with undetectable minimal residual disease (uMRD) at a sensitivity level of 10-4 (MRD4). uMRD had to be demonstrated in two consecutive peripheral blood or bone marrow aspirate tests conducted ≥ 12 weeks apart. In patients who did not meet these criteria, zanubrutinib was continued as monotherapy beyond cycle 28 until confirmed uMRD. In addition to del(17p) and/or TP53 mutation, 85 % of patients had unmutated IGHV, and a complex karyotype with ≥ 3 or ≥ 5 abnormalities was present in 50 % and 36 % of cases, respectively. To date, only small percentages of patients have met the early stopping criteria due to short follow-up.
Zanubrutinib plus venetoclax showed favorable safety and tolerability. COVID-19 was the most common treatment-emergent adverse event (TEAE; any grade, 55 %), followed by diarrhea (39 %), nausea (30 %), contusion (29 %), fatigue (23 %), neutropenia (22 %) and arthralgia (15 %). Grade ≥ 3 TEAEs included neutropenia (17 %), diarrhea (9 %) and COVID-19 (3 %). Among TEAEs of special interest, infections ranked first due to the high COVID-19 rate (any grade, 71 %; grade ≥ 3, 15 %), while hemorrhage ranked second (any grade, 54 %; grade ≥ 3, 2 %) and neutropenia third (any grade, 22 %; grade ≥ 3, 17 %). Second primary malignancies occurred in 13 % (grade ≥ 3, 8 %). Low rates were reported regarding hypertension (any grade, 10 %; grade ≥ 3, 8 %) and atrial fibrillation/flutter (2 %). The zanubrutinib lead-in gave rise to a 91 % reduction in the proportion of patients at high risk of tumor lysis syndrome (TLS).
Zanubrutinib plus venetoclax induced deep and durable remissions after a median follow-up of 31.6 months. All of the 65 response-evaluable patients responded, with 48 % achieving CR/CRi (Figure 1). The rates of uMRD in the peripheral blood increased with longer treatment duration. At the time of this interim analysis, best uMRD rates were 59 % in ≥ 1 peripheral blood sample and 37 % in ≥ 1 bone marrow sample. Median progression-free survival (PFS) had not been reached; at 12 and 24 months, 95 % and 94 % of patients, respectively, were alive and progression-free. Results in patients who meet the MRD-guided early stopping rules will be reported as data mature. The ongoing phase III CELESTIAL-TNCLL trial is evaluating fixed-duration therapy with zanubrutinib and the next-generation BCL2 inhibitor sonrotoclax in patients with treatment-naïve CLL [3].
Figure 1: Response rates obtained with zanubrutinib plus venetoclax
Pirtobrutinib plus venetoclax/obinutuzumab
Venetoclax plus obinutuzumab in addition to the non-covalent BTK inhibitor pirtobrutinib is currently being investigated as a limited-duration triplet combination in a phase II study containing untreated patients with CLL/SLL. While pirtobrutinib is taken orally through 13 cycles, obinutuzumab is administered in cycles 1-6 and venetoclax in cycles 2-13 with ramp-up in cycle 2. Patients with detectable MRD at ≥ 10-5 in either peripheral blood or bone marrow at the end of cycle 13 can continue pirtobrutinib plus venetoclax for an additional 12 cycles. The uMRD4 rate in the bone marrow after cycle 7 is defined as the primary endpoint of the study. Jain et al. presented the first results for 40 patients after a median follow-up of 11.7 months [4]. At that time, 20 individuals had completed cycle 13.
One cycle of pirtobrutinib and obinutuzumab prior to the initiation of venetoclax enabled downgrading of the TLS risk to the medium- or low-risk categories in most patients with high or medium risk. The triple combination induced very high uMRD rates at a sensitivity level of 10-6 (Figure 2). At the end of cycle 7, 79 % and 64 % of patients had obtained uMRD in the peripheral blood and bone marrow, respectively. These rates increased further to 90 % and 85 %, respectively, at the end of cycle 13. They are numerically higher than those achieved with ibrutinib and venetoclax in the blood marrow after 6 and 12 months (33 % and 52 %, respectively) [5]. At the time of the analysis, no patient had progressed or died.
Pirtobrutinib and venetoclax dose reductions were necessary in 30 % each, with neutropenia being the most common reason. Grade 3-4 neutropenia and thrombocytopenia occurred in 60 % and 18 %, respectively. Fifty-five percent of patients required G-CSF prophylaxis. One patient developed atrial fibrillation, which was grade 2. The trial is currently enrolling into a 40-patient expansion cohort.
Figure 2: Pirtobrutinib plus venetoclax and obinutuzumab: MRD results at serial time points
MRD findings for ibrutinib/venetoclax
The phase II ERADIC trial is comparing the standard fludarabine, cyclophosphamide and rituximab (FCR) regimen to an MRD-guided approach using the combination of ibrutinib and venetoclax in patients with intermediate risk in whom FCR is less effective. The intermediate-risk cohort is defined by either unmutated IGHV status, 11q deletion or complex karyotype in the absence of TP53 alteration. While arm 1 received 6 cycles of FCR Q4W, arm 2 was treated with ibrutinib for 3 cycles followed by the combination including an initial venetoclax ramp-up. After 9 months, patients with MRD < 0.01 % in the bone marrow continue ibrutinib plus venetoclax for another 6 months and stop at month 15. Those with MRD ≥ 0.01 % at month 9 continue ibrutinib and venetoclax for 18 months and stop at month 27 irrespective of their clinical response or MRD status. The primary endpoint is the percentage of patients with MRD < 0.01 % in the bone marrow at month 27. Each study arm contains 60 individuals. Interim results of MRD kinetics after a median follow-up of 29.7 months were presented at EHA 2024 [6].
The MRD analysis revealed a clear increase in the uMRD rates on ibrutinib plus venetoclax therapy between months 9 and 21, suggesting that a 9-month treatment course is too short to achieve a very good MRD < 10-4 response. At 9 months, the uMRD rates in the bone marrow were 38 % vs. 69 % for ibrutinib plus venetoclax vs. FCR. In the peripheral blood, this was 57 % vs. 78 %; at month 15, the uMRD rates were equal (78 % vs. 77 %), and at 21 months, the rate for ibrutinib plus venetoclax exceeded that for FCR (82 % vs. 66 %). Sixteen out of 20 patients with uMRD in the peripheral blood at 9 months discontinued treatment at 15 months according to the trial design. Two more discontinuations took place at month 21, and treatment was continued in two patients with partial response based on investigator’s choice. Only two conversions to detectable MRD were identified at month 27. In the group that had not achieved uMRD in the peripheral blood at month 9, 21 patients continued treatment. Eight of these converted to uMRD at month 15, with only one showing a detectable MRD result again at month 21. Clinically, CR/CRi was present in 66 % with ibrutinib plus venetoclax vs. 56 % with FCR at 9 months. Overall, these results support the use of MRD kinetics for treatment decision-making.
However, toxicity remained an important factor in both arms. Grade ≥ 3 serious AEs included TLS (n = 5 vs. 3 with ibrutinib plus venetoclax vs. FCR), COVID 19 (4 each), febrile neutropenia (0 vs. 5) and atrial fibrillation (2 vs. 0), among others. Infections were generally more common with FCR, whereas cardiovascular disorders emerged more frequently with the targeted approach. One case each of colorectal cancer and basal cell carcinoma was reported as grade ≥ 3 secondary malignancies in the experimental arm. In the control arm, one patient developed MDS and another AML which led to death in both cases. Another patient treated with FCR died of septic shock. Three deaths occurred in the group that received ibrutinib and venetoclax (2 sudden deaths, 1 COVID-19–related death).
The authors noted that toxicity should be taken into account when determining whether treatment should be continued because of detectable MRD in the bone marrow at month 9. Upcoming data of the primary endpoint analysis of the study at month 27 will be highly relevant with a view to determining the best strategy.
CAPTIVATE: results in high-risk subgroups
First-line treatment with ibrutinib plus venetoclax was evaluated in the phase II CAPTIVATE study in two cohorts. The fixed-duration (FD) cohort was treated with 12 cycles of the combination after 3 cycles of ibrutinib lead-in. In the MRD-guided cohort, patients with uMRD after the regimen described for the FD cohort were randomized to either placebo or ibrutinib. Upon disease progression, ibrutinib-based treatment could be reinitiated in both cohorts. At EHA 2024, Jacobs et al. reported outcomes in patients from the FD cohort who had high-risk genomic features [7]. Also, retreatment outcomes were presented for the FD cohort and the placebo arm of the MRD-guided cohort.
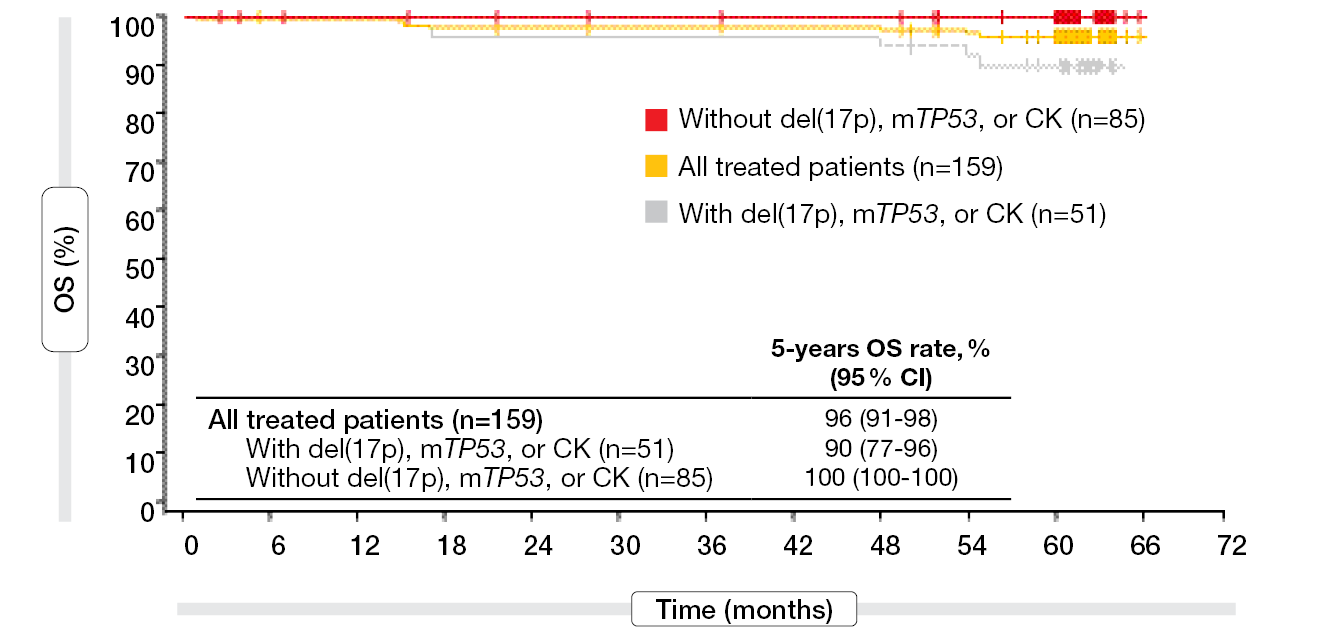
A total of 159 patients received the combination in the FD cohort. Median PFS had still not been reached in this group after a follow-up of 5.5 years, and the 5-year PFS rate was 67 %. In patients with del(17p), mutant TP53, or complex karyotype (n = 51), the 5-year PFS rate was 54 %, and in those without these genomic high-risk features (n = 85), 77 %. Irrespective of other risk factors, the group with unmutated IGHV was shown to have a lower 5-year PFS rate than that with mutated IGHV (56 % vs. 80 %). After exclusion of patients with del(17p), TP53 mutation or complex karyotype, the respective rates were 68 % and 85 %, which demonstrated the impact of these risk features on PFS. Moreover, 5-year PFS was improved in patients who achieved uMRD4 in the peripheral blood or bone marrow. In the high-risk genomic subgroups, the 5-year PFS rates were consistently higher in those with uMRD4 at 3 months after the end of treatment than in those without MRD4. For overall survival (OS), the analysis yielded 5-year rates ≥ 90 % regardless of genomic risk features (Figure 3). The authors noted that fixed-duration ibrutinib plus venetoclax confers meaningful survival benefits in patients with high-risk genomics.
Among 61 patients with disease progression after completion of fixed-duration ibrutinib plus venetoclax, 32 initiated retreatment with single-agent ibrutinib (n = 25) or ibrutinib plus venetoclax (n = 7). Unmutated IGHV and del(17p)/mutant TP53 were present in these groups in 78 % and 31 %, respectively. Thirty-four percent had a complex karyotype and bulky lymph node disease ≥ 5 cm. In spite of this high-risk setup, retreated patients showed promising responses with overall response rates (ORRs) of 86 % and 71 % in the groups receiving single-agent ibrutinib (22 evaluable patients) and ibrutinib plus venetoclax, respectively. CRs were observed in 5 % and 14 %, respectively. The AEs reported with ibrutinib-based retreatment were consistent with the known safety profiles of ibrutinib monotherapy and the combination. As the authors concluded, based on the safety profiles of fixed-duration ibrutinib plus venetoclax and ibrutinib-based retreatment, this approach appears to offer a favorable benefit-risk ratio.
Figure 3: High 5-year overall survival rates observed for ibrutinib plus venetoclax irrespective of the genomic risk features del(17p), TP53 mutation and complex karyotype (CK)
Zanubrutinib vs. ibrutinib/venetoclax
In the absence of head-to-head clinical studies comparing zanubrutinib with venetoclax plus ibrutinib in the setting of treatment-naïve CLL, Munir et al. conducted a matching-adjusted indirect comparison (MAIC) using individual patient-level data from the SEQUOIA study that were matched against aggregate data from the GLOW and CAPTIVATE trials [8]. Due to the lack of common control arms linking SEQUOIA with GLOW or CAPTIVATE, two unanchored MAICs were performed. The zanubrutinib population from Cohort 1 of the SEQUOIA study (arm A) was reweighted to match the key characteristics of the ibrutinib plus venetoclax population in GLOW. At the same time, the pooled zanubrutinib population from Cohort 1 and 2 of SEQUOIA (arms A and C) was reweighted to match the key characteristics of the ibrutinib plus venetoclax population in CAPTIVATE.
While no statistically significant difference in investigator-assessed PFS was demonstrated for zanubrutinib vs. ibrutinib plus venetoclax, the population-adjusted estimate revealed a potential trend in favor of zanubrutinib (HR, 0.78). Also, the safety profile of zanubrutinib was significantly better than that of the combination despite longer treatment exposure (median, 43-44 months vs. 13.8 months). Multiple AEs showed significantly lower incidence with zanubrutinib, in particular diarrhea, neutropenia, nausea, anemia, atrial fibrillation, decreased appetite, and arthralgia. These differences might be considered at the time of treatment decision-making.
Relapsed/refractory CLL
Prolonged venetoclax/ibrutinib induction: SAKK 34/17
Studies exploring ibrutinib plus venetoclax typically incorporate a brief initial ibrutinib treatment phase to mitigate the TLS risk, with treatment discontinuation after 12 months. However, uMRD rates obtained in these trials have often fallen short of expectations [9-11]. The SAKK 34/17 trial was conducted to evaluate the efficacy of a prolonged 24-month induction phase with ibrutinib plus venetoclax to maximize the uMRD and CR/CRi rates. Furthermore, the scientists chose a longer ibrutinib lead-in period of 6 cycles. In cycle 7, the venetoclax dose was stepped up, and ibrutinib plus venetoclax was administered from cycle 8 to 31. Finally, this study assessed whether circulating tumor DNA (ctDNA) captures the genetics and residual disease from tissue-restricted CLL clones, and whether ctDNA can identify resistance mutations early on during the treatment period. uMRD with CR/CRi at the end of cycle 30 was defined as the primary endpoint. Patients who were MRD-negative and had achieved CR/CRi at that time were observed for up to 5 years. On the other hand, if MRD persisted, ibrutinib plus venetoclax was continued for up to 5 years until MRD negativity, CR/CRi, progression, or intolerance.
According to the findings reported for 30 patients at EHA 2024, the study met its primary endpoint. Forty percent of patients showed uMRD with CR/CRi at cycle 30 [12]. This rate exceeded the rates obtained in trials with 1 year of ibrutinib plus venetoclax exposure [9, 10]. However, a higher discontinuation rate of 37 % indicates a trade-off. After 6 cycles of ibrutinib lead-in, the percentage of patients at low TLS risk shifted from 33 % to 50 %. One TLS event occurred (3.3 %) that was grade 3. The PFS rate at cycle 31 was 85.5 % after a median follow-up of 42 months, with median PFS not having been reached.
The analysis of cell-free DNA did not enable early detection of resistance mutations, and the measurements did not add any information to immunoglobulin high-throughput sequencing. Moreover, no clinical mutations beyond those detected in CLL cells from the peripheral blood were revealed by the ctDNA assessments.
Pirtobrutinib in BTKi-naïve patients
Reversible BTK inhibition with pirtobrutinib monotherapy is being assessed in various B-cell malignancies in the phase I/II BRUIN study. Results for 35 BTK-inhibitor–naïve patients with relapsed/refractory CLL/SLL were presented at EHA 2024 by Eyre et al. [13]. The median number of prior lines of systemic therapy in this cohort was 2. Almost half had bulky lymphadenopathy ≥ 5 cm and complex karyotype. Eighty percent showed unmutated IGHV, and TP53 mutations and/or del(17p) were present in 37 %.
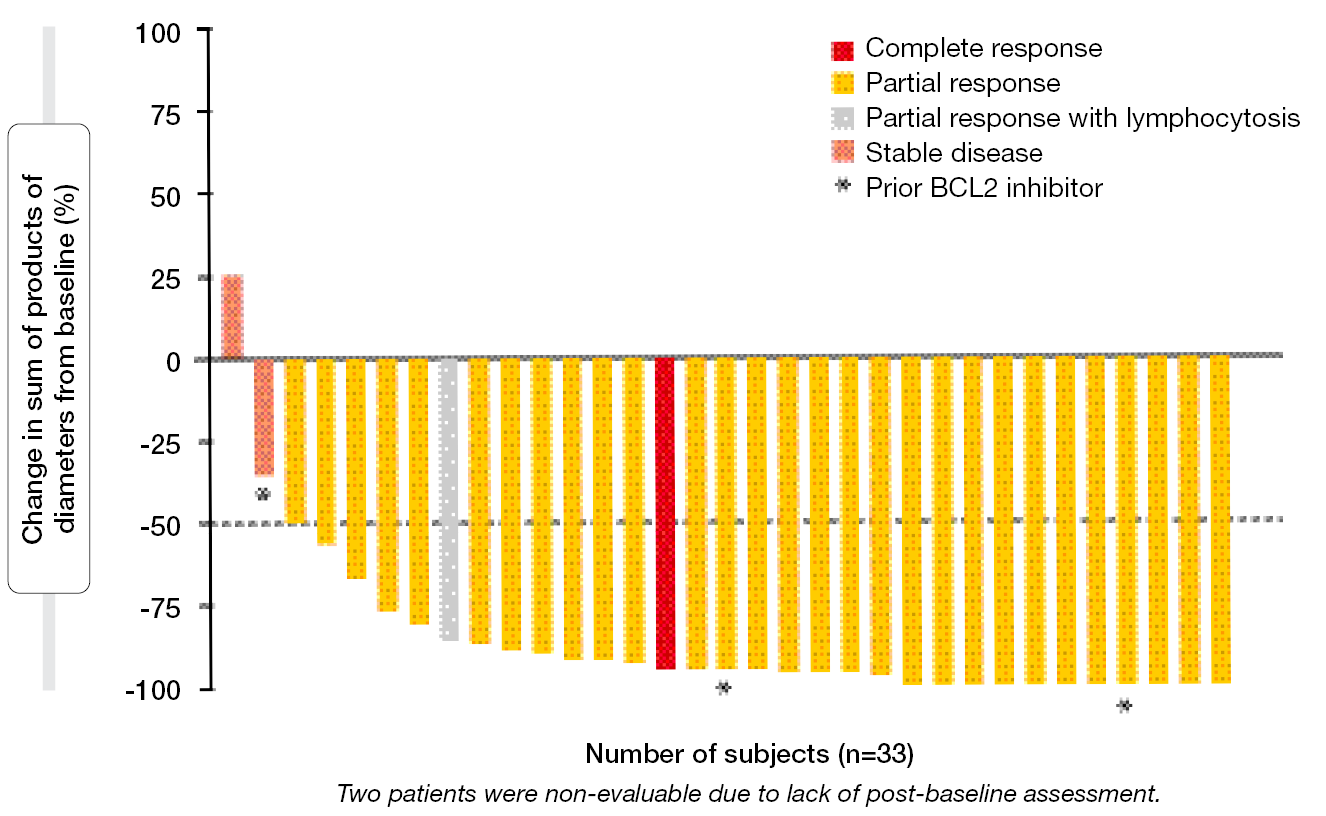
The ORR achieved with pirtobrutinib monotherapy was as high as 91.4 % and 94.3 % according to the independent review committee (IRC) and the investigators, respectively. Almost all patients experienced substantial reductions in tumor burden (Figure 4). One patient developed CR (2.9 %). ORRs for the population with genomic high-risk features were favorable, ranging from 85.7 % to 100 %. Neither median PFS nor median OS had been reached after a median follow-up of approximately 30 months. At 24 months, 88.0 % of patients were alive, and 74.7 % were progression-free according to IRC.
The safety profile of pirtobrutinib in BTK-inhibitor–naïve patients resembled that observed in the overall BRUIN cohort. Treatment-related AEs (TRAEs) mainly comprised neutropenia (40.0 %), diarrhea (20.0 %), contusion (14.3 %), and anemia (11.4 %). Among TRAEs of interest, infections were most common (any grade, 25.7 %; grade ≥ 3, 2.9 %), followed by rash (17.1 %) and bruising (14.3 %). Except for infections, no TRAEs of interest were graded as ≥ 3. The analysis revealed low rates of dose reduction and discontinuation due to TRAEs (11.4 % and 5.7 %, respectively). Phase III trials are currently evaluating pirtobrutinib monotherapy in treatment-naïve patients with CLL/SLL (BRUIN CLL-313; NCT05023980) and patients who are treatment-naïve or pretreated with non-BTK inhibitor therapy (BRUIN CLL-314; NCT05254743).
Figure 4: Reductions in tumor burden with pirtobrutinib monotherapy
ALPINE: PRO-based prediction of progression
The randomized phase III ALPINE study has demonstrated significantly increased PFS for zanubrutinib compared to ibrutinib in patients with relapsed/refractory CLL [14]. Zanubrutinib is the only BTK inhibitor to date that has shown PFS superiority vs. ibrutinib in this setting. Serrano et al. conducted analyses based on patient-reported outcomes (PRO) from 601 patients in ALPINE with the aim of developing a joint model to examine the association between the time to recurrent PRO-based deterioration and disease progression [15]. Investigator-assessed PFS was analyzed as the terminal event measure.
After predicting PFS from the risk of recurrent symptomatic deterioration events and using a joint model to adjust for baseline stratification factors and change from baseline in corresponding symptoms, zanubrutinib remained superior to ibrutinib regarding disease progression in the ALPINE trial. The analysis demonstrated that leading predictors for the risk of disease progression included recurrent symptomatic deterioration in appetite, diarrhea, and dyspnea. Accordingly, patient reporting of deterioration in these symptoms might indicate a need for increased clinical monitoring.
Statistical comparisons in the relapsed setting
Multilevel network meta-regression is a newly developed method that facilitates the estimation of relative treatment effects between interventions for different target populations based on networks of any size. Shadman et al. used this method to estimate the treatment effect of zanubrutinib relative to acalabrutinib and ibrutinib in patients with relapsed/refractory CLL based on the ALPINE and ELEVATE-RR trials [16]. ELEVATE-RR has established the non-inferiority of acalabrutinib compared to ibrutinib in relapsed/refractory CLL, with lower rates of atrial fibrillation, hypertension and bleeding [17].
The results indicated that zanubrutinib conferred significantly longer PFS compared to acalabrutinib and ibrutinib in a population akin to that of the ALPINE trial as well as ELEVATE-RR patients who were characterized by high-risk cytogenetics. Zanubrutinib also demonstrated potential improvement in OS versus both ibrutinib and acalabrutinib, although these results were not statistically significant, possibly due to the limitations in the number of studies available and their sample sizes.
Another group of investigators used MAIC to evaluate the relative efficacy of zanubrutinib and acalabrutinib in the relapsed/refractory CLL setting [18]. Individual patient-level data from ALPINE were matched against aggregate data from the ASCEND trial that has revealed PFS improvement with acalabrutinib vs. rituximab plus idelalisib/bendamustine [19, 20]. Due to the lack of a common comparator arm, an unanchored MAIC was used.
After matching, investigator-assessed PFS was significantly improved for zanubrutinib vs. acalabrutinib (HR, 0.68; p = 0.0448), while OS was potentially improved (HR, 0.60; p = 0.0575). The CR rates favored zanubrutinib in both unadjusted (OR, 2.88; p = 0.0198) and base case-adjusted (OR, 2.90; p = 0.0270) populations. These results were robust across multiple sensitivity analyses. However, as the authors pointed out, randomized controlled trials remain the gold standard for evaluating evidence of relative efficacy.
7-year update for obinutuzumab/ibrutinib/venetoclax
Longer-term outcomes are still being assessed for combination regimens containing a BTK inhibitor and BCL2 inhibitor with or without an anti-CD20 antibody. Rogers et al. presented the 7-year follow-up for a phase II study exploring ibrutinib plus venetoclax and obinutuzumab in treatment-naïve (TN) and relapsed/refractory (RR) CLL/SLL [21]. The patients had been accrued in one RR cohort and two TN cohorts (n = 25 each). While the RR cohort completed accrual in 2017 and had a median follow-up of 83.0 months, TN1 and TN2 completed accrual in 2016 and 2019, respectively, and had median follow-up times of 85.6 and 51.7 months, respectively. Treatment consisted of 8 cycles of obinutuzumab, ibrutinib from cycle 2, and venetoclax from cycle 3 including initial ramp-up. After 14 cycles, the treatment was stopped. Response assessment occurred 2 months after completion of cycle 14 (end of treatment, EOT).
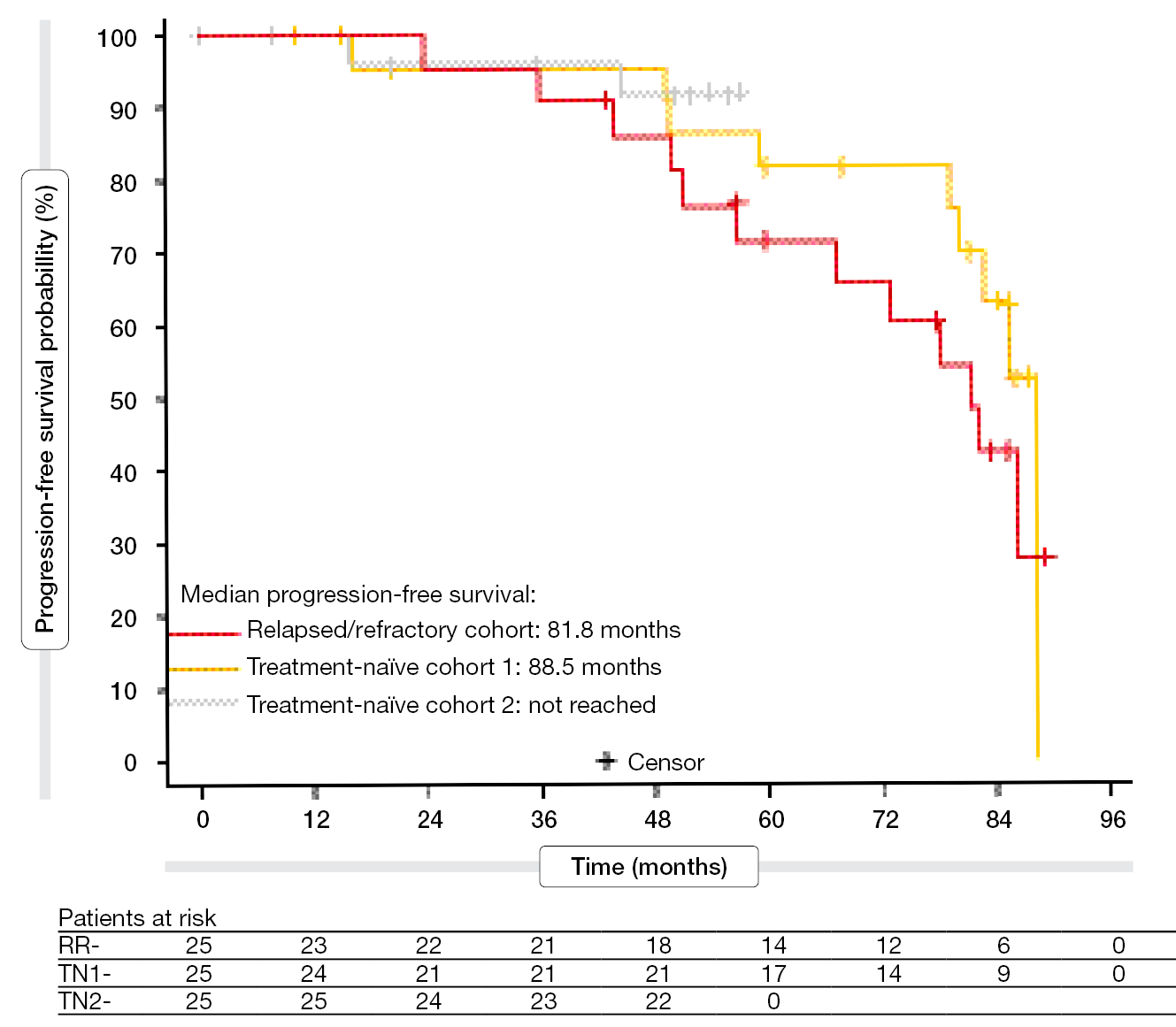
At EOT, the ORR was 88 % for the RR cohort and 90 % for the TN cohorts. In the RR group, 44 % had achieved uMRD; for TN1 and TN2, this was 56 % and 60 %, respectively. Median PFS had been reached for the RR and TN1 cohorts (81.8 and 88.5 months, respectively; Figure 5). In the TN2 group, 91 % of patients were alive and progression-free at 48 months. Unexpectedly, PFS after treatment did not differ according to the MRD status at EOT. Median OS had not been reached in any cohort. The 60-month OS rates were 95.0 % and 90.9 % in the RR and TN1 groups, respectively.
Characteristics associated with lower PFS and decreased probability of achieving uMRD were non-specific markers of more aggressive CLL biology such as increases in beta-2-microglobulin and lactate dehydrogenase. No mutations showing associations with either PFS or uMRD status were identified. After treatment, 10.7 % of patients developed grade ≥ 3 infections. COVID-19 of any grade occurred in 29.3 % (grade ≥ 3, 4 %). Second neoplasms were observed in 25.3 %; among these, 71 % were non-melanoma skin cancers. Notably, no second myeloid malignancies emerged. Infections constituted the most common cause of death. Overall, nine patients died, with all of the fatalities occurring after completion of active treatment. Additional studies are required to determine the benefit of 3-drug regimens vs. 2-drug regimens, mechanisms of resistance and sensitivity, and the optimal sequence of treatments over the lifespan of CLL patients.
Figure 5: Ibrutinib plus venetoclax and obinutuzumab: 7-year progression-free survival
ASSURE: safety of acalabrutinib
The ongoing global, open-label phase IIIB safety study ASSURE is examining acalabrutinib monotherapy in patients with CLL in a real-world setting. ASSURE contains three cohorts including TN patients, those with RR disease, and ibrutinib-intolerant individuals who had previously discontinued ibrutinib therapy for any reason apart from disease progression. Acalabrutinib is being administered for 48 cycles and beyond in patients still benefiting from it. Opat et al. reported interim safety results for 310 and 202 patients in the TN and RR groups, respectively, and for 40 ibrutinib-intolerant patients [22].
At 30 months, the proportions of those who were still receiving treatment in these three groups were 71 %, 62 % and 45 %, respectively. The safety profile of acalabrutinib was in keeping with the observations from previous clinical studies. Rates of cardiac events were low. Among events of clinical interest, atrial fibrillation/flutter occurred in 4.7 % (grade ≥ 3, 1.6 %) in the total group, while hemorrhage was observed in 48.9 % (grade ≥ 3, 3.8 %) and hypertension in 7.8 % (grade ≥ 3, 3.3 %). Substantial proportions of patients with atrial fibrillation or hypertension had a prior history of these conditions.
Grade ≥ 3 TEAEs were reported in 57 % of patients overall (TN, 54 %; RR, 63 %; ibrutinib-intolerant, 45 %). TEAEs led to treatment discontinuation in 19 % (TN, 15 %; RR, 26 %; ibrutinib-intolerant, 18 %). Seventy-five percent of patients had infections including COVID-19 (grade ≥ 3, 29.9 %). Deaths due to TEAEs occurred in 56 patients, with the majority dying from COVID-19 and related complications. Second primary malignancies excluding non-melanoma skin cancer were reported in 8.9 % (grade ≥ 3, 4.5 %).
Hypertension on BTK inhibitor treatment
As is known, BTK inhibitor therapy can elicit cardiovascular AEs such as hypertension. A retrospective cohort study based on the Symphony Health Solutions Database sought to describe the risk of new-onset and worsening hypertension in 30,559 patients with newly diagnosed CLL who received treatment with (n = 2,392) or without (n = 28,167) BTK inhibitors [23]. The baseline prevalence of hypertension was 73 % and 72 % in the BTK inhibitor and non-BTK inhibitor cohorts, respectively.
Patients in the BTK inhibitor cohort, as compared to the non-BTK inhibitor cohort, had a higher rate of new-onset hypertension (inverse probability treatment weighting [IPTW] HR, 1.50) or worsening hypertension (IPTW HR, 1.16) within 1 year of treatment initiation. Comparatively more patients in the BTK inhibitor cohort increased their number of antihypertensive drug classes (19.5-24.8 %) than patients in the non-BTK inhibitor cohort (14.2-17.5 %). These data suggest that development of hypertension is an important consideration in the long-term management of patients with CLL undergoing BTK inhibition.
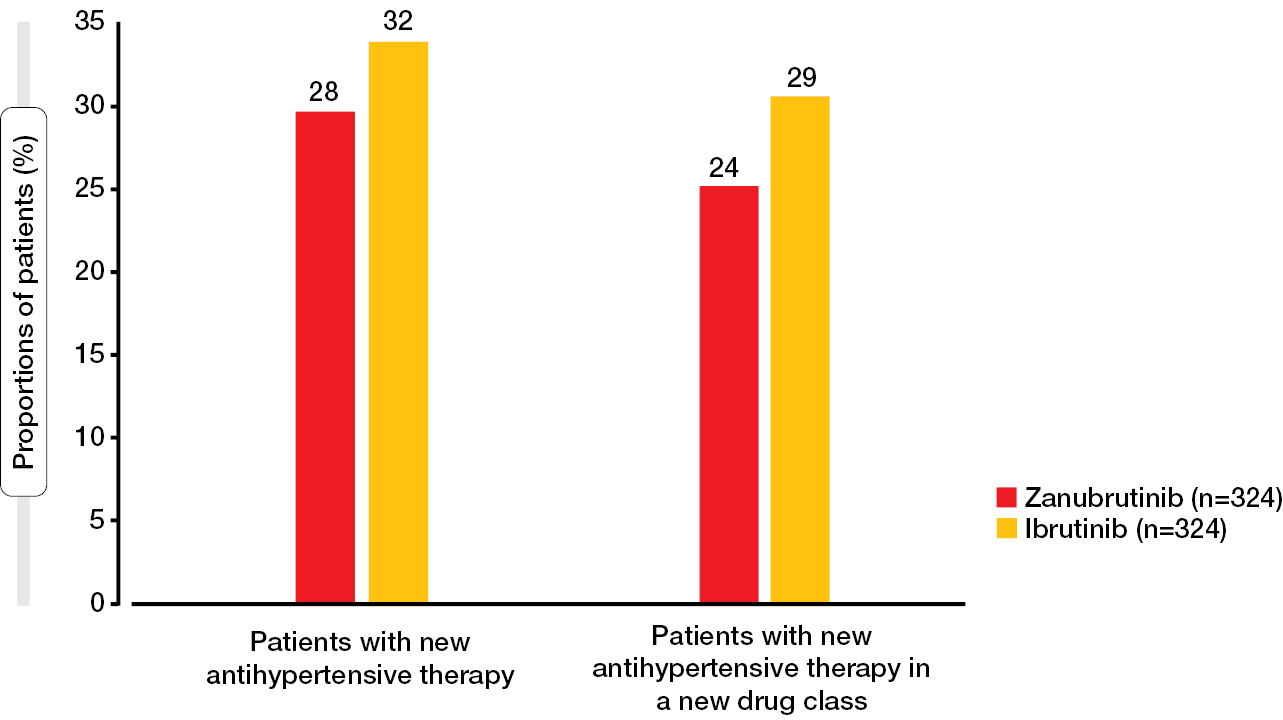
In the ALPINE trial, treatment-emergent hypertension rates were similar across the zanubrutinib and ibrutinib arms, which is not consistent with other clinical studies that have demonstrated lower hypertension rates with zanubrutinib [14, 24, 25]. Therefore, a post-hoc analysis evaluated the risk of developing hypertension with zanubrutinib vs. ibrutinib in ALPINE based on the initiation of antihypertensive therapy reported in this study. According to the results, smaller proportions of zanubrutinib-treated patients started new antihypertensive drugs or new classes of antihypertensives compared to those treated with ibrutinib (Figure 6) [26]. Time to initiation of the first antihypertensive drug in a new class was significantly longer with zanubrutinib (HR; 0.72; p < 0.05), and for the time to initiation of the first new antihypertensive drug, the analysis showed a trend favoring zanubrutinib (HR, 0.77; p = 0.071). As the authors noted, these findings should be considered when starting BTK inhibitor therapy in patients with CLL/SLL who have an elevated cardiovascular risk.
Figure 6: Proportions of patients who required new antihypertensive medication in the ALPINE study
BTKi use in US community oncology practices
Real-word results in a total of 6,875 CLL/SLL patients who initiated BTK inhibitor treatment in the USA between January 2020 and July 2023 were analyzed by Hou et al. with the aim of describing treatment patterns and AEs [27]. The investigators used the Integra Connect database of electronic health records, practice management, and claims data from 55 practices and > 1,600 providers from the community oncology setting. While 2,815 patients started BTK inhibitor treatment in the first line, 249 did so in later lines. In the first-line setting, ibrutinib was most commonly used (49.3 %), followed by acalabrutinib (43.4 %) and zanubrutinib (7.2 %). In later lines, acalabrutinib ranked first (43.4 %), followed by ibrutinib (39.8 %) and zanubrutinib (16.9 %).
Safety and efficacy outcomes were better for acalabrutinib and zanubrutinib than for ibrutinib. Significantly more patients experienced cardiovascular AEs in the first-line setting with ibrutinib vs. acalabrutinib and zanubrutinib at month 6 (12.1 % vs. 7.6 % and 7.3 %, respectively; p < 0.05) as well as month 9 (14.6 % vs. 9.4 % and 8.5 %, respectively; p < 0.05). Median time to treatment discontinuation or death in the first line was shortest with ibrutinib (13.7 vs. 19.2 and 19.3 months, respectively). Zanubrutinib showed improved time to next treatment (TTNT) in the first line compared to ibrutinib and acalabrutinib (median TTNT, not reached vs. 30.2 and 35.8 months, respectively). Additional research is required to explain and validate the observed differences favoring zanubrutinib over acalabrutinib.
A retrospective study assessed real-world switching and sequencing to the next line of treatment in US-based patients initiating BTK inhibition as their first-line or second-line CLL/SLL therapy [28]. Information on 2,816 and 1,253 individuals starting their first- and second-line treatment, respectively, was obtained from the Integra Connect database. Ibrutinib was the most commonly used first-line agent (50.5 % vs. 44.0 % and 5.6 % for acalabrutinib and zanubrutinib, respectively), whereas acalabrutinib therapy prevailed in the second line (53.6 % vs. 37.8 % and 8.54 % for ibrutinib and zanubrutinib, respectively). Compared to the groups treated with the other BTK inhibitors, the zanubrutinib cohort had the highest mean age in the second line (p = 0.0037) and the highest percentages of patients with SLL across the lines (p < 0.0001).
Regardless of the line of therapy, the switching rates at ≤ 60 days and 61-89 days were significantly lower for zanubrutinib than for ibrutinib and acalabrutinib (p < 0.0001, Figure 7). This resulted in significantly lower proportions of patients receiving their next line of treatment at 180 days in the first-line setting (13.9 % for zanubrutinib vs. 21.1 % and 24.5 % for ibrutinib and acalabrutinib, respectively; p < 0.0001) and in the second-line setting (9.1 % vs. 29.2 % and 18.6 %; p < 0.0001). The authors emphasized that longer follow-up and larger zanubrutinib sample size are required for a comprehensive assessment of outcomes associated with the use of BTK inhibitors in CLL/SLL.
Figure 7: Switching rates within 90 days for acalabrutinib, ibrutinib and zanubrutinib in clinical practice
REFERENCES
- Tam CS et al., Zanubrutinib monotherapy for patients with treatment naïve chronic lymphocytic leukemia and 17p deletion. Haematologica 2021; 106(9): 2354-2363
- Ma S et al., Combination of zanubrutinib + venetoclax for treatment-naïve CLL/SLL with del(17p) and/or TP53: Preliminary results from SEQUOIA arm D. EHA 2014, abstract S160
- Patten P et al., CELESTIAL-TNCLL: An ongoing, open-label, multiregional, phase 3 study of sonrotoclax (BGB-11417) + zanubrutinib vs venetoclax + obinutuzumab for treatment-naïve CLL. EHA 2024, abstract PB2540
- Jain N et al., Combined pirtobrutinib, venetoclax, and obinutuzumab in first-line treatment of patients with chronic lymphocytic leukemia (CLL): A phase 2 trial. EHA 2024, abstract S164
- Jain N et al., Combined ibrutinib and venetoclax for first-line treatment of patients with chronic lymphocytic leukemia (CLL): 5-year follow-up data. Blood 2023; 142 (Supplement 1): 4635
- Quinquenel A et al., Minimal residual disease-guided combination of ibrutinib and venetoclax compared to FCR in untreated patients with CLL of intermediate risk. Interim results of MRD kinetics in the ERADIC trial from the FILO group. EHA 2024, abstract S161
- Jacobs R et al., Outcomes in high-risk subgroups after fixed-duration ibrutinib + venetoclax for chronic lymphocytic leukemia/small lymphocytic lymphoma: Up to 5.5 years of follow-up in the phase 2 CAPTIVATE study. EHA 2024, abstract P675
- Munir T et al., Efficacy and safety of zanubrutinib versus venetoclax+ibrutinib in treatment-naïve chronic lymphocytic leukemia: A matching-adjusted indirect comparison. EHA 2024, abstract P702
- Hillmen P et al., Ibrutinib plus venetoclax in relapsed/refractory chronic lymphocytic leukemia: The CLARITY study. J Clin Oncol 2019; 37(30): 2722-2729
- Kater AP et al., Minimal residual disease-guided stop and start of venetoclax plus ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia (HOVON141/VISION): primary analysis of an open-label, randomised, phase 2 trial. Lancet Oncol 2022; 23(6): 818-828
- Wierda WG et al., Ibrutinib plus venetoclax for first-line treatment of chronic lymphocytic leukemia: Primary analysis results from the minimal residual disease cohort of the randomized phase II CAPTIVATE study. J Clin Oncol 2021; 39(34): 3853-3865
- Condoluci A et al., Ibrutinib lead-in followed by venetoclax plus ibrutinib in patients with relapsed/refractor chronic lymphocytic leukemia (SAKK 34/17 trial). EHA 2024, abstract 667
- Eyre TA et al., Pirtobrutinib in relapsed/refractory CLL/SLL: Results from BTKi naïve cohort in the phase 1/2 BRUIN study. EHA 2024, abstract P656
- Brown JR et al., Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 2023; 388(4): 319-332
- Serrano D et al., Patient-reported outcome-based recurrent symptomatic deterioration predicts disease progression: Results from the ALPINE trial. EHA 2024, abstract P1834
- Shadman M et al., Zanubrutinib versus other Bruton tyrosine kinase inhibitors in relapsed/refractory chronic lymphocytic leukemia: A multilevel network meta-regression. EHA 2024, abstract P698
- Byrd JC et al., Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: Results of the first randomized phase III trial. J Clin Oncol 2021; 39(31): 3441-3452
- Shadman M et al., Efficacy of zanubrutinib versus acalabrutinib in the treatment of relapsed or refractory chronic lymphocytic leukemia: A matching-adjusted indirect comparison. EHA 2024, abstract P700
- Ghia P et al., ASCEND: Phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol 2020; 38(25): 2849-2861
- Ghia P et al., Acalabrutinib versus investigator’s choice in relapsed/refractory chronic lymphocytic leukemia: Final ASCEND trial results. Hemasphere 2022; 6(12): e801
- Rogers KA et al., 7-year update on a phase 2 trial of fixed-duration obinutuzumab, ibrutinib, and venetoclax for CLL. EHA 2024, abstract S162
- Opat S et al., Interim results from ASSURE: A phase 3b safety study of acalabrutinib in patients with chronic lymphocytic leukemia. EHA 2024, abstract P684
- Douglas Kou T et al., Risk of new-onset or worsening hypertension in patients with newly diagnosed chronic lymphocytic leukemia treated with BTK inhibitors: A real-world study using the Symphony Health Solutions Database. EHA 2024, abstract P1874
- Tam CS et al., A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood 2020; 136(18): 2038-2050
- Tam CS et al., Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol 2022; 23(8): 1031-1043
- Ramirez D et al., Risk of hypertension in patients with CLL/SLL who participated in ALPINE: A post hoc analysis. EHA 2024, abstract P1836
- Hou JZ et al., Real-world Bruton tyrosine kinase inhibitor treatment patterns and outcomes among patients with chronic lymphocytic leukemia or small lymphocytic lymphoma in US community oncology practices. EHA 2024, abstract P685
- Pinilla-Ibarz J et al., Real-world treatment switching and sequencing to next line of therapy of zanubrutinib, acalabrutinib, and ibrutinib in CLL/SLL. EHA 2024, abstract P697
© 2024 Springer-Verlag GmbH, Impressum
More posts
Relative efficacy of several treatment options in marginal zone lymphoma
Chemoimmunotherapy (CIT), immunotherapy and chemotherapy regimens are commonly used for the treatment of patients with marginal zone lymphoma. Moreover, the BTK inhibitor zanubrutinib has shown activity in the relapsed/refractory setting based on the phase II, single-arm MAGNOLIA and BGB-3111-AU-003 trials [1, 2]. Walewska et al. conducted a matching-adjusted indirect comparison (MAIC) to estimate the comparative efficacy of these treatment strategies in relapsed/refractory marginal zone lymphoma [3].
Follicular lymphoma: news on bispecific antibody treatment
In the setting of relapsed/refractory follicular lymphoma (FL), progression-free survival (PFS) deteriorates with successive relapses, which implies a high unmet need for therapies that can improve disease control and extend survival after relapse [1]. The Fc-silenced CD20 x CD3 bispecific antibody odronextamab is being investigated in patients with relapsed/refractory B-cell malignancies in the multicohort, multicenter, phase II ELM-2 study.
Innovative BCL2 inhibition: indications fanning out across B-cell malignancies
The combination of the first-generation BCL2 inhibitor venetoclax and the first-in-class BTK inhibitor ibrutinib has demonstrated efficacy in patients with chronic lymphocytic leukemia (CLL) [1], although tolerability of this regimen is limited. Next-generation agents can be expected to provide optimized toxicity profiles. The second-generation BCL2 inhibitor sonrotoclax inhibits BCL2 in a more selective and pharmacologically potent manner than venetoclax, with a shorter half-life preventing drug accumulation that might contribute to toxicity [2].
BTK degraders: emerging activity in various B-cell malignancies
The new class of BTK degraders is being developed in response to emerging patterns of resistance that limit the utility of BTK and BCL2 inhibitors. On one hand, BTK mutations decrease the efficacy of both covalent and non-covalent BTK inhibitors; on the other hand, some mutations lead to “kinase dead” or “kinase bypassing” BTK mutants with intact B-cell receptor signaling through a scaffolding function of BTK [1, 2].
Meeting unmet needs in mantle cell lymphoma
In older or unfit patients with mantle cell lymphoma (MCL), bendamustine plus rituximab (BR) is the most common first-line therapy, while intensive regimens are usually unsuitable in this population even though they provide durable responses [1, 2]. The addition of the first-in-class BTK inhibitor ibrutinib to first-line BR has been shown to prolong progression-free survival (PFS) in the SHINE trial [3].
Reducing risks further in chronic lymphocytic leukemia
The second-generation BTK inhibitor zanubrutinib is being tested in the phase III SEQUOIA trial in the setting of untreated chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) with and without del(17p). Zanubrutinib monotherapy has shown high tolerability and efficacy in arm C of the study that included patients with del(17p) [1].