Ensartinib outperforms crizotinib as a treatment for patients with ALK-positive NSCLC who were naïve to ALK-targeted therapy
eXalt3 was a randomized, open-label, comparator-controlled phase III trial designed to evaluate the efficacy and safety of treatment with either of two anaplastic lymphoma kinase (ALK) inhibitors alone, ensartinib or crizotinib, in the treatment of advanced or metastatic ALK-positive non-small cell lung cancer (NSCLC) patients. The trial enrolled patients with an ALK-positive, stage IIIB or IV NSCLC confirmed either by local FDA-approved assays or central confirmation via fluorescence in situ hybridization (FISH). Although patients with previous ALK inhibitor treatment were excluded, patients with ECOG performance status of 0 to 2 with ≤1 prior chemotherapy regimen were allowed. Patients were stratified according to prior chemotherapy status, ECOG performance status, geographical location, and brain metastases at baseline.
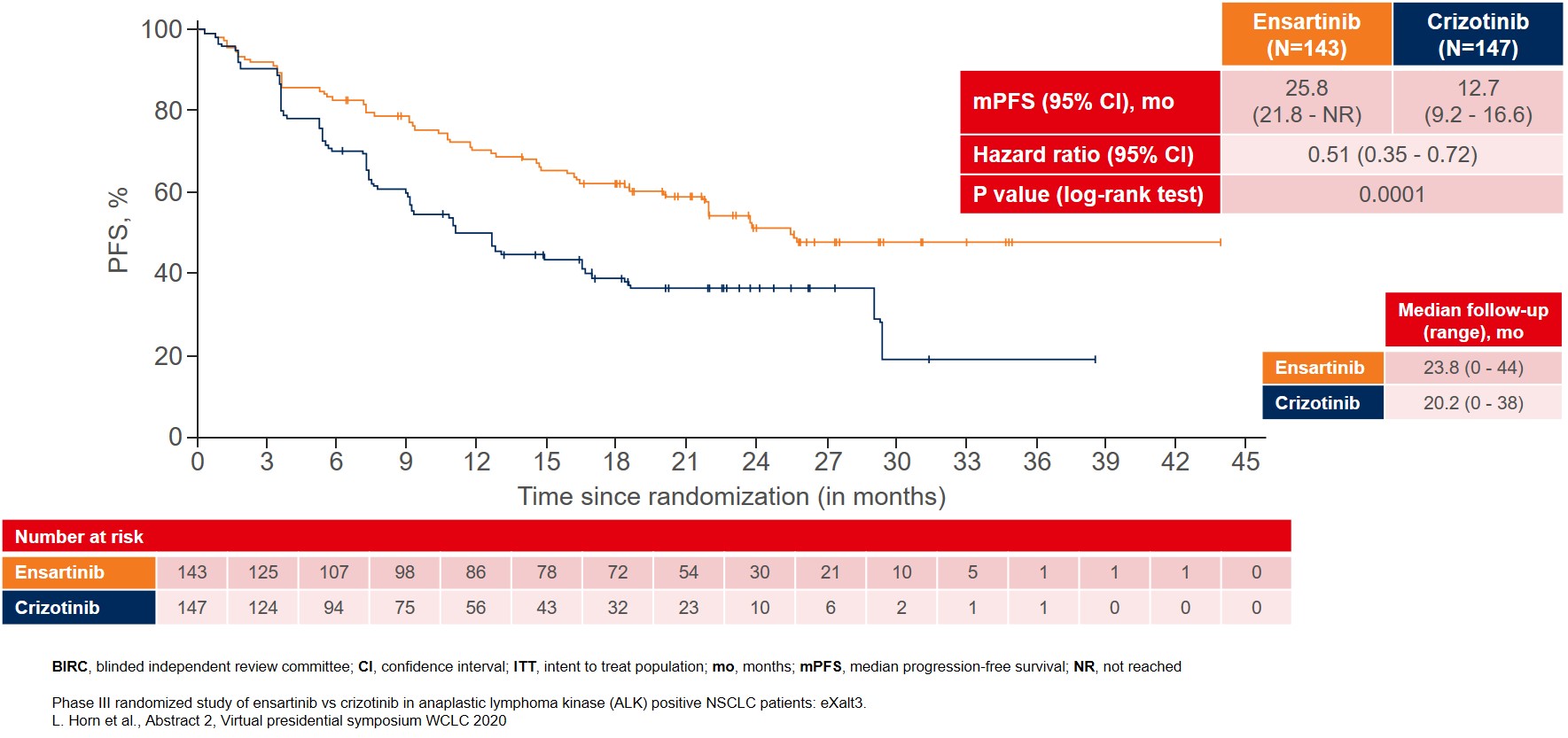
A total of 290 patients were randomized 1-to-1 to receive either ensartinib daily or crizotinib twice per day until disease progression. No crossover between study arms was allowed during the trial. The primary endpoint was blinded and an independent review committee (BIRC) assessed median progression-free survival (mPFS) in the Intention-to-treat (ITT) population.
The baseline characteristics were similar in both treatment groups with around one-third of patients presenting brain metastases at baseline (33% vs 39%) or having prior chemotherapy regimen (24% vs 29%), and a higher proportion of never smokers (59% vs 64%) as compared to current or former smokers (41% vs 36%).
The interim analysis showed that the primary endpoint was met with BIRC-assessed mPFS for patients treated with ensartinib where mPFS improved to 25.8 months as compared to 12.7 months for patients treated with crizotinib (Figure 1).
Figure 1: BIRC-assessed median PFS in the ITT population as the primary endpoint of the eXalt3 study
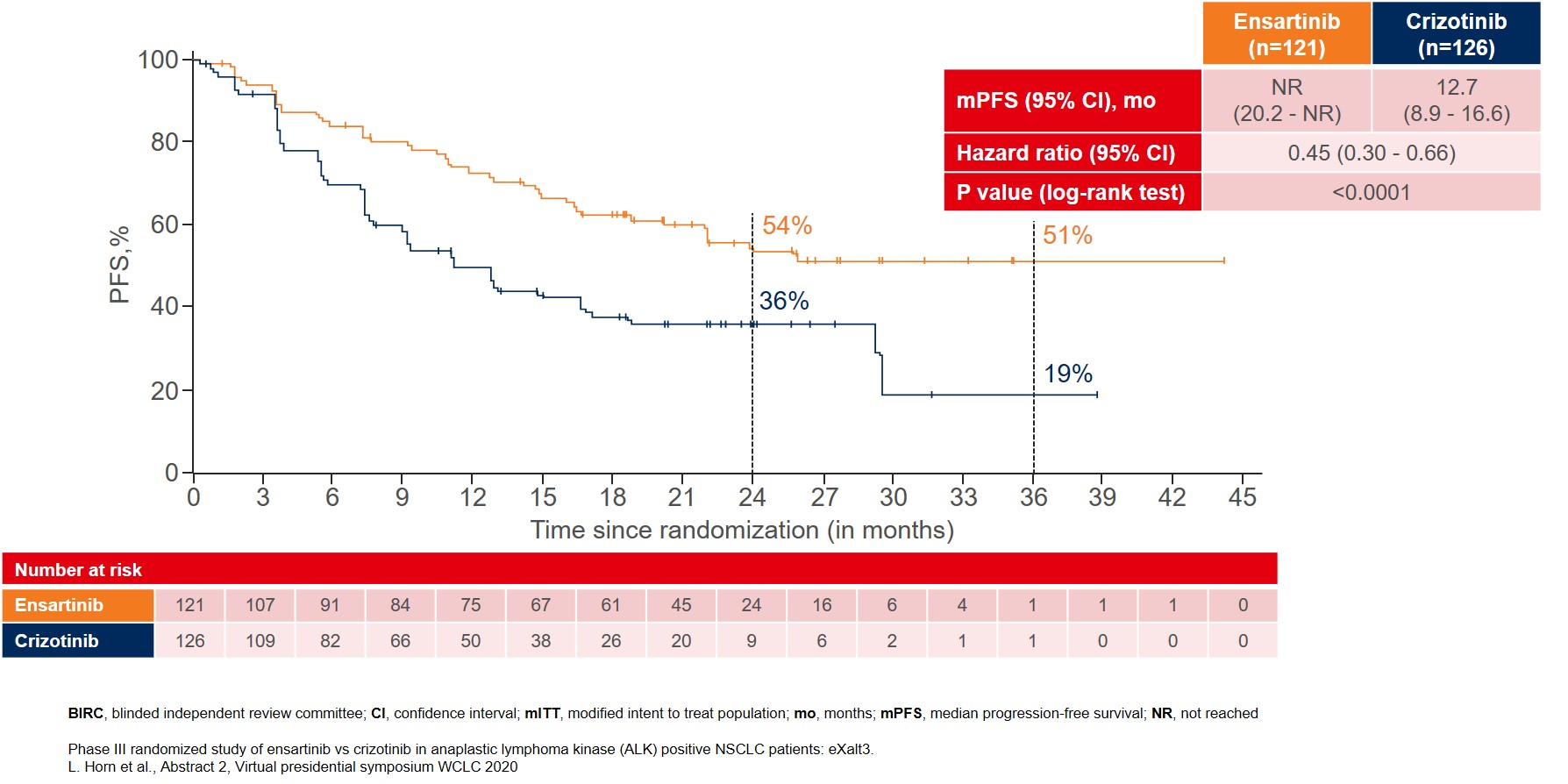
More remarkable improvement in mPFS was reported in the modified ITT (mITT) population, which included patients with centrally confirmed ALK gene rearrangements by FISH testing. In this mITT population (n=247), mPFS was not reached (NR) for ensartinib as compared to 12.7 months for crizotinib (Figure 2).
Figure 2: BIRC-assessed median PFS in the modified ITT population with patients that were centrally confirmed for ALK gene rearrangements by FISH testing
Other efficacy endpoints also showed improvement, e.g. objective response rate (ORR) (75% vs 67%) and duration of response (DOR) (NR vs 27.3 months), in favor of ensartinib as compared to crizotinib. The overall survival (OS) data were still immature, with the median OS not reached for either of the treatment arms, with around 20% of events documented at the time of analysis in both treatment arms. Sub-group analysis of patients without brain metastases at baseline (n=157) in the mITT population showed that ensartinib significantly delayed time to treatment failure (TTF) as compared to crizotinib (4.2 vs 23.9 months; HR 0.32, 95% CI 0.15-0.64; p = 0.0011).
The safety profile for ensartinib was reported to be as tolerable as crizotinib with similar rates of serious treatment-related adverse events (TRAEs) (8% vs 6%), TRAEs leading to dose reduction (24% vs 20%) and TRAEs leading to drug discontinuation (9% vs 7%). The most common TRAEs for patients on ensartinib were skin toxicities including rash and pruritis as well as elevation of liver transaminases, most of which were grade 1 or 2. The most common TRAEs for the crizotinib group were consistent with previous studies such as elevation of liver transaminases, gastrointestinal toxicities and edema.
With longer follow-up, ensartinib shows a positive trend towards improved mPFS, representing a new first-line treatment option for patients with ALK-positive NSCLC.
More posts
Nivolumab as a new option in patients with relapsed malignant mesothelioma
Until recently, no randomized phase III trials have demonstrated OS improvement in patients with relapsed malignant mesothelioma. PD-1 inhibition with single-agent nivolumab has shown activity in three phase II studies, one of which led to the approval of nivolumab in Japan.
Interview: Lung cancer screening: hurdles in daily routine and in the research laboratory
All of us agree that LDCT is effective and should be widely implemented. I would say that cost and awareness are the two major issues that are impeding the implementation of LDCT all over the world. Cost-effectiveness of LDCT has been demonstrated by many publications; and there are even other arguments in favor of the cost-effectiveness of this technology.
Immunotherapy: combination regimens and new data on the significance of mutations
Substantial OS and PFS improvements have led to the implementation of the regimen evaluated in the KEYNOTE-189 trial as standard first-line approach for stage IV non-squamous NSCLC without sensitizing EGFR/ALK alterations.
What is new in SCLC?
A novel approach for targeting lung tumors with small-cell histology consists in the inhibition of transactivated transcription, as small-cell lung cancer (SCLC) has been found to be a transcription-addicted malignancy. Rudin et al. defined four molecular SCLC subtypes according to their differential expression of four key transcription regulators.
Interview: Antibody-drug conjugates: the age of almost unlimited possibilities has just begun
Antibody-drug conjugates have opened up an entirely new paradigm. Targeted therapy requires specific mutations, and immunotherapy only works if the tumor expresses neoantigens or is essentially able to respond to these agents. As we know, these two approaches do not work forever, not every patient responds to them, and certainly not every patient has a targetable mutation.
Specific treatment approaches in the EGFR-mutated setting
EGFR exon 20 insertion mutations are found in approximately 5 % to 12 % of EGFR-mutated NSCLC tumors, i.e., in 2 % of all NSCLC cases. They represent the third most common EGFR mutation after L858R and exon 19 deletion. However, EGFR TKIs cannot be used to treat lung cancer with exon 20 insertions as they are insensitive to these drugs due to steric hindrance at the TKI-binding site.