Conventional chemotherapy combined with PD-1/PD-L1
Neoadjuvant camrelizumab plus albumin-bound paclitaxel and cisplatin
Neoadjuvant chemotherapy (CT), which has been the standard-of-care for resectable NSCLC, resulted only in modest survival benefits of approximately 5 %. However, CT combined with neoadjuvant immunotherapy is a promising strategy in improving survival outcomes of patients with resectable NSCLC [1]. Hence, several small single-arm phase II studies are ongoing in this setting, all including patients with stage III disease [2-4].
Camrelizumab has previously demonstrated a survival benefit in patients with advanced NSCLC when combined with CT in a first-line setting of advanced NSCLC [5, 6]. At this year’s ESMO IO meeting, Jie Lei reported on the final analysis of a phase II trial (NCT04338620) evaluating the neoadjuvant combination of camrelizumab – an anti-PD-1 antibody – and CT in resectable NSCLC patients [7].
This randomized, controlled, multicenter trial was conducted in treatment-naïve patients diagnosed with resectable stage IIIA or IIIB (T3 N2 M0) NSCLC (according to the 8th edition of the AJCC/UICC TNM staging system), aged 18 to 70 years, presenting with an ECOG performance status of 0 or 1. Patients were randomized (1:1) to receive either three cycles of camrelizumab (200 mg, IV, Q3W) plus three cycles of CT (albumin-bound paclitaxel plus carboplatin [AUC 5 at Day one] or plus cisplatin [75 mg/m2 at Day 1] or plus nedaplatin [100 mg/m2 at Day 1]) or three cycles of CT alone. Four to six weeks post-treatment, all patients had surgery. The primary study endpoint was the complete pathologic response (pCR) – defined as no residual viable tumor cells in both the primary tumor and sampled lymph node-, while MPR, ORR, EFS and safety were secondarily analyzed.
A total of 88 patients received neoadjuvant treatment (n = 43 for camrelizumab plus CT and n = 45 for CT alone). The median age was 61 years, and the majority of patients was male. The most frequent tumor type was squamous cell carcinoma (62.8 % in the camrelizumab + CT arm and 71.1 % in the CT arm, respectively) and most of the patients had clinical stage IIIA (69.8 % in the camrelizumab + CT arm and 80.0 % in the CT arm, respectively). Regarding surgery, most patients had a lobectomy (90 % in the camrelizumab + CT arm and 81 % in the CT arm, respectively). An R0 resection was achieved in 92.5 % and 85.7 % of the patients, respectively. The median duration from final treatment to surgery was 4.7 versus 4.6 weeks, respectively.
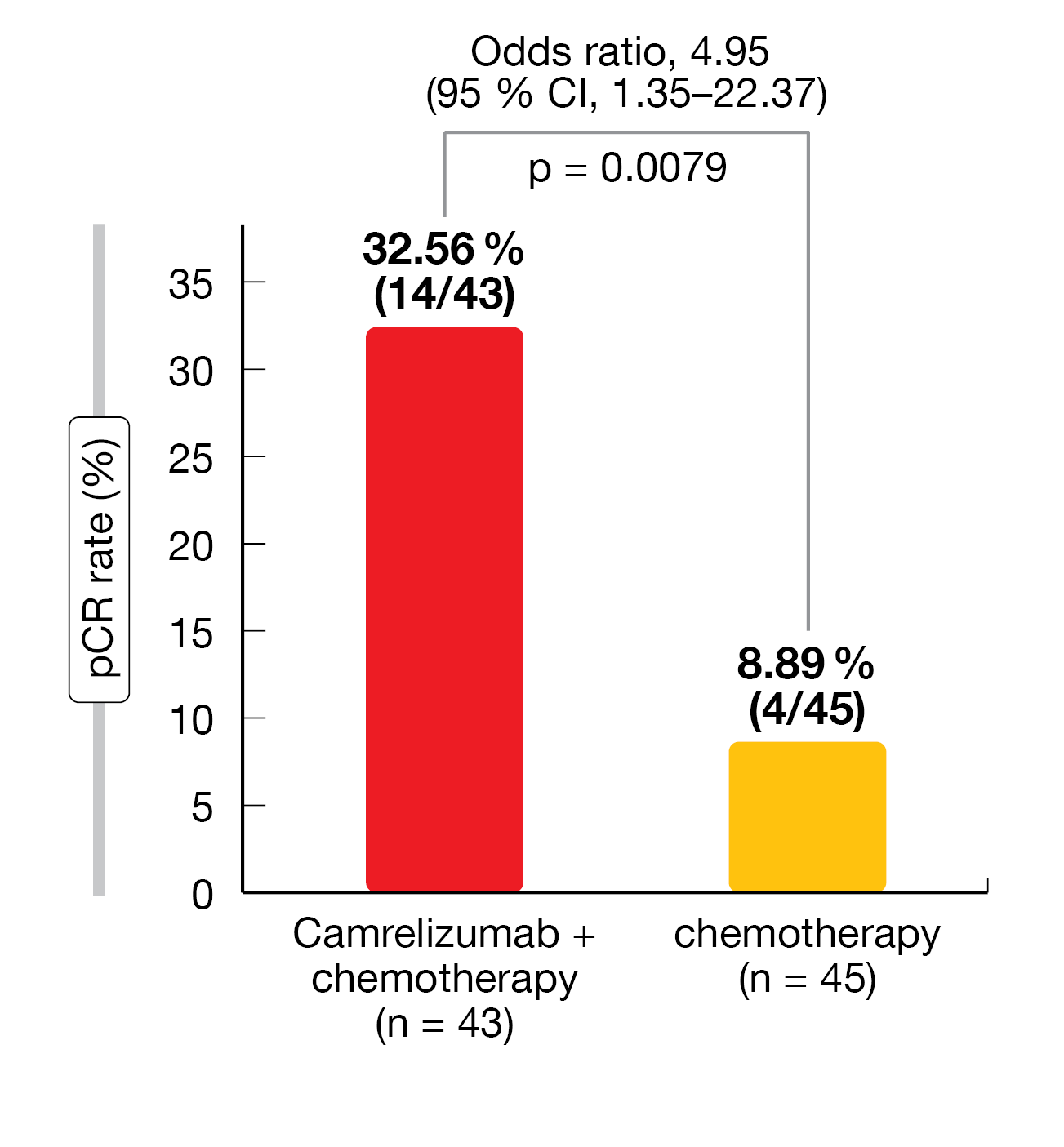
Overall, neoadjuvant camrelizumab plus CT was associated with a significantly higher pCR rate (32.56 % vs 8.89 %; odds ratio [OR] = 4.95; 95 % CI, 1.35-22.37; p = 0.0079) and MPR rate (65.12 % vs 15.56 %; OR = 10.13; 95 % CI, 3.32-32.76; p < 0.0001) compared with CT alone (Figure 1). At the time of data cut-off (August 31, 2022), the median EFS and DFS were not reached; however, there was a trend in favor of camrelizumab plus CT over CT alone (24-month EFS rate = 76.9 % vs 67.6 %; 24-month DFS rate = 78.4 % vs 71.7 %). More patients responded to the treatment in the camrelizumab + CT group than in the CT group (ORR = 72.09 % vs 53.33 %; OR = 2.26; 95 % CI: 0.85-6.08; p = 0.0814) and a higher number of patients achieved a CR (CR: 11 vs 4 patients, respectively; PR: 20 patients in both groups).
The overall incidence of grade ≥ 3 TRAEs in the modified intention-to-treat (ITT) population was 25.6 % in the camrelizumab plus CT arm and 11.1 % in the CT arm. The most frequent grade ≥ 3 TRAEs were a decrease in white blood cell count (14.0 % vs 4.4 %, respectively) and a decrease in neutrophil count (7.0 % vs 11.1 %). Immune-related AEs (irAEs) < grade 3 occurred in 53.5 % of the patients, the most frequent ones being reactive cutaneous capillary hyperplasia (RCCEP, 44.2 %), hypothyroidism (7 %) and hyperthyroidism (2.3 %). Of note, a grade ≥ 3 surgery-related AE – a perioperative death following a cardiovascular accident – occurred in one patient treated with camrelizumab plus CT.
These data demonstrated that combining neoadjuvant camrelizumab plus CT was superior to CT alone in terms of pCR and MPR in resectable patients with stage IIIA or IIIB NSCLC. The safety profile was acceptable, with no new signals observed. This combination proved to be an effective and safe new potential neoadjuvant treatment option for this patient population.
Figure 1: Complete pathologic response (pCR) rate with neoadjuvant camrelizumab plus chemotherapy versus chemotherapy in patients with resectable NSCLC (modified ITT population)
RATIONALE-307 trial: 1L tislelizumab plus chemotherapy
Tislelizumab is an anti PD-1 monoclonal antibody with reduced Fcγ receptor binding specificity on macrophages [8]. The combination of tislelizumab with a platinum-based CT has been assessed in the open-label phase III RATIONALE-307 trial (NCT03594747) as first-line treatment for patients with advanced squamous NSCLC. In this context, a previously reported interim analysis showed a significantly prolonged PFS and higher tumor response rates with this combination compared to CT alone [9]. At the ESMO IO 2022 meeting, Jie Wang presented updated results from the final analysis of the RATIONALE-307 trial after a median of 10.1 months additional follow-up since the last interim cut-off [10].
Patients included in this study had treatment naïve stage IIIB (not eligible to curative surgery or radiotherapy) or IV squamous NSCLC. They were equally randomized to receive either tislelizumab (200 mg, IV, Q3W) plus four to six cycles of paclitaxel and carboplatin (Arm A) or tislelizumab (200 mg, IV, Q3W) plus nab-paclitaxel and carboplatin (Arm B), or four to six cycles of paclitaxel and carboplatin only (Arm C). Tislelizumab was administered for 1 hour on D1 of cycles 1 and 2 and for 30 minutes in subsequent infusions. Tislelizumab treatment continued every three weeks until lack of clinical benefit or intolerable toxicity. Doublet chemotherapy was given until completion of 4 to 6 cycles (at the investigator’s discretion), occurrence of disease progression, or intolerable toxicity, whichever occurred first. Patients in arm C could cross over to receive tislelizumab monotherapy if it was determined that they had independent review committee (IRC)–confirmed disease progression [11]. The primary endpoint was the PFS as assessed by an independent review committee (IRC) in the ITT population. The OS, ORR as assessed by an IRC, DoR and safety were secondary endpoints.
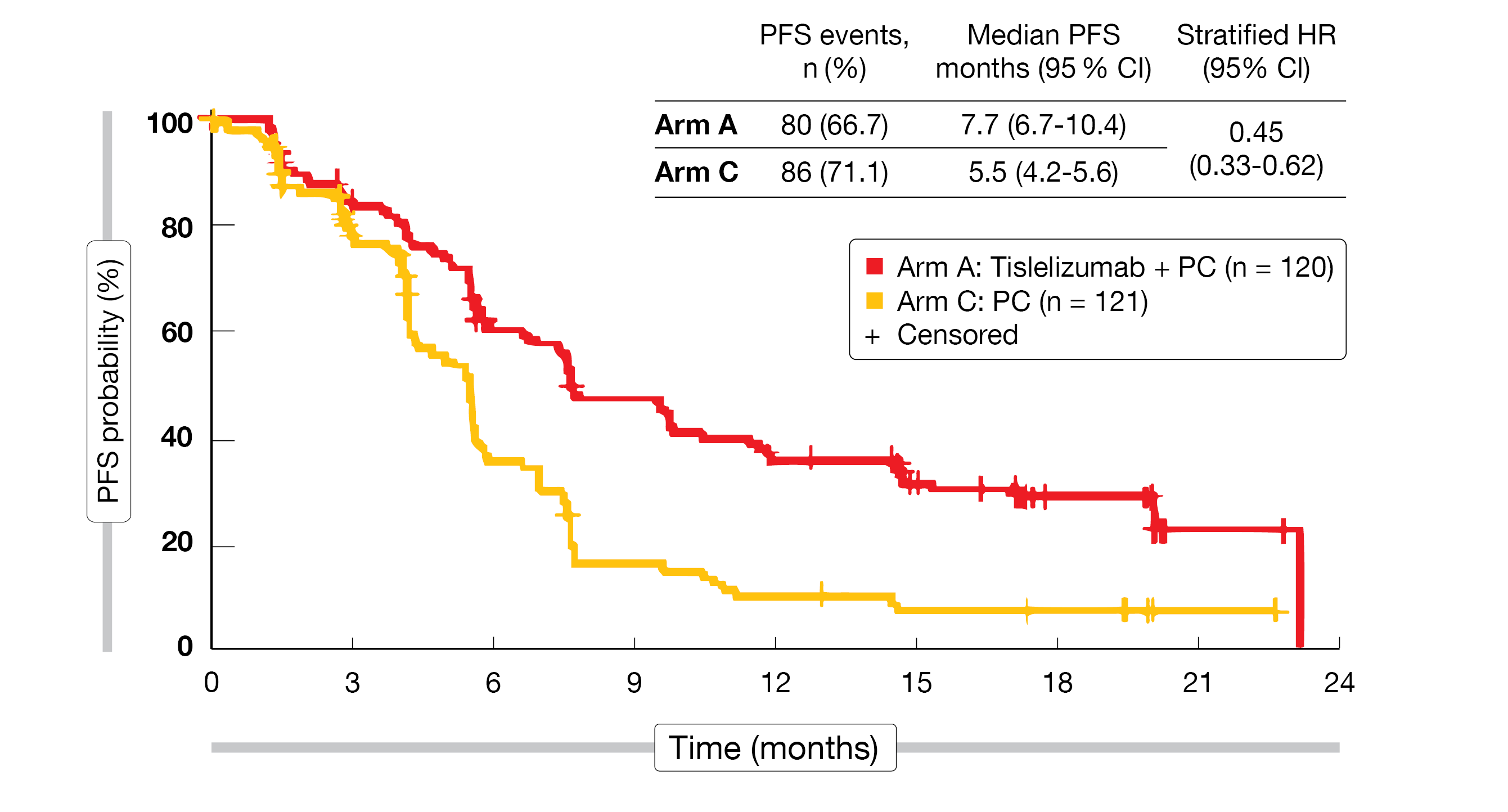
At the time of data cut-off (September 30, 2020), a total of 360 patients had been randomized (Arm A, n = 120; Arm B, n = 119; Arm C, n = 121). The overall median age was 62 years, 91.7 % were male and 2/3 had a stage IV disease at baseline. In total, 38.3 % had a PD-L1 expression rate of less than 1 %, and 34.7 % a rate of 50 % or more. The median study follow-up was 18.7 months. At the time of data cut-off, 25.8 % of the patients were still on treatment in Arm A and 28.6 % in Arm B, while all patients in Arm C had completed their four to six cycles of chemotherapy. The interim analysis had previously demonstrated a PFS superiority of the combined treatments (Arms A and B) over chemotherapy alone (Arm C). At the final analysis cut-off, the benefit of tislelizumab plus CT over CT alone in terms of PFS was maintained in both Arms A and B (median PFS in Arm A vs Arm C: 7.7 vs 5.5 months; HR = 0.45; 95 % CI, 0.33-0.62 (Figure 2) and in Arm B vs Arm C: 9.6 vs 5.5 months; HR = 0.43; 95 % CI, 0.31-0.60). The outcome was consistent in all PD-L1 expression subgroups. More patients in Arms A and B compared to Arm C responded to the treatment (ORR = 74.2 % and 73.9 %, vs 47.9 %, respectively), achieved a CR (5.8 % and 6.7 % vs 0.8 %) and had a prolonged median DoR (8.4 months and 8.6 months vs 4.3 months). The ORR benefit of tislelizumab plus CT versus CT alone was also observed in all analyzed PD-L1 expression subgroups.
According to the final analysis, 63.6 % of patients in Arm C received subsequent immunotherapy (92.2 % of them received tislelizumab) after a median time of 10.3 weeks after the last CT cycle. In contrast, a subsequent treatment with immunotherapy was required by 15.0 % of patients in Arm A and 10.9 % of patients in Arm B, only.
Safety data were similar to that of the interim analysis and no new safety signals were reported. Overall, the combination of tislelizumab plus CT proved to be tolerable.
With a longer follow-up, the final analysis of RATIONALE-307 trial demonstrated that the addition of tislelizumab to chemotherapy is clinically beneficial as first-line treatment of advanced squamous NSCLC versus chemotherapy in terms of PFS, ORR and DoR.
Figure 2: Progression-free survival (PFS) of patients with advanced squamous NSCLC receiving tislelizumab plus CT (Arm A) versus CT alone (Arm C) in the RATIONALE-307 trial.
AK105-302 study: 1L penpulimab plus carboplatin and paclitaxel
Penpulimab, a novel humanized IgG1 monoclonal antibody directed against PD-1, has been engineered to eliminate Fcγ receptor binding, and therefore to reduce antibody-dependent cellular toxicity and phagocytosis. In 2021, penpulimab received its first approval in China for the second-line treatment of relapsed or refractory classic Hodgkin’s lymphoma [12]. Penpulimab is currently investigated in a phase III trial (NCT03866993) in combination with carboplatin and paclitaxel for the first-line treatment of patients with locally advanced or metastatic squamous NSCLC. At ESMO IO 2022, Baohui Han presented the final analysis of this randomized, double-blind, placebo-controlled, multicenter trial [13].
Eligible patients had a histologically or cytologically confirmed diagnosis of stage IIIB-IV squamous NSCLC, no EGFR, ALK or ROS 1 mutations, at least one measurable tumor lesion according to RECIST v1.1, an ECOG performance status or 0 or 1 and any rate of PD-L1 expression. Patients were randomized 1:1 to receive four cycles of CT (paclitaxel [175 mg/m2, IV] and carboplatin [AUC 5, IV] Q3W) plus either penpulimab (200 mg, IV, Q3W) or placebo (IV, Q3W) on Day 1 of each cycle, followed by penpulimab or placebo as maintenance therapy until disease progression or for a maximum of 24 months. Of note, patients in the placebo arm were allowed to cross-over to penpulimab monotherapy (open-label) at any time during the 24-month study duration. The primary endpoint was PFS, as assessed by an IRC in the ITT population and in the PD-L1 positive population (TPS PD-L1 ≥ 1 %). Overall survival, ORR, and DoR, as assessed by an independent regulatory review commission (IRRC) and by the investigators as well as the PFS evaluated by the investigators, were secondarily analyzed. Patients were stratified by PD-L1 expression status and gender.
A total of 175 patients were enrolled in each study group. The median age was 68 years and most of them were males (92 %). Overall, 18 % of the patients had a PD-L1 expression status over 50 %. Patients who received penpulimab plus CT had a significantly longer PFS than patients treated with placebo plus CT (7.6 months vs 4.2 months; HR = 0.44; 95 % CI, 0.34-0.56; p < 0.0001). The 12-month and 24-month PFS rates were also more prominent in the penpulimab plus CT group than in the placebo plus CT group (37.1 % vs 9.2 % and 23.8 % vs 5.9 %, respectively). A PFS-benefit of adding penpulimab to CT was also demonstrated in the PD-L1 positive population (8.1 months vs. 4.2 months; HR = 0.37; 95 % CI, 0.27-0.51; p < 0.0001). At the time of data cut-off (June 1, 2022), the median follow-up was 23.56 months. Although the median OS was not reached, there was an obvious trend in favor of penpulimab plus CT (NR vs 19.8 months; HR = 0.55; 95 % CI, 0.40-0.75; p = 0.0002) and a significantly higher 24-month OS rate in this group compared to placebo plus CT (61.1 % vs 41.6 %). More patients responded to the treatment in the penpulimab plus CT arm (ORR, 71.4 % vs 44.0 %) and the response was also more durable for these patients (mDoR, 8.25 months vs 2.96 months).
The incidence of grade ≥ 3 TRAEs was 63.6 % in the penpulimab plus CT group and 62.9 % in the placebo plus CT group. The incidence of serious TRAEs was 28.3 % vs 26.9 %, respectively. In total, 5.2 % vs 3.4 %, respectively, of patients experienced TRAEs leading to treatment discontinuation.
Combining penpulimab with CT demonstrated to be an effective and well-tolerated first-line treatment option for patients with advanced squamous NSCLC. This trial is the second randomized study (after investigating tislelizumab in the RATIONALE-307 trial [9]) suggesting that the abrogation of Fcγ receptor interactions may lead to higher response rates and lower toxicity.
EMPOWER-LUNG 3 trial: 1L cemiplimab plus CT plus ipilimumab
Cemiplimab is a monoclonal antibody directed against PD-1, which has been first developed, investigated, and approved for the treatment of advanced cutaneous squamous cell carcinoma [14]. When administered as monotherapy, cemiplimab demonstrated (NCT03088540) its survival benefit over chemotherapy in the EMPOWER-Lung 1 trial [15]. Thus, it was approved in the USA and the European Union as first-line treatment for patients with advanced NSCLC, PD-L1 ≥ 50 % and without EGFR, anaplastic ALK or ROS1 genomic aberrations. Moreover, in patients with low PD-L1 expression, the combination of ipilimumab – an anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) antibody – and CT has been shown to potentialize the effects of anti–PD-1 therapy. At this year’s ESMO IO meeting, Ana Baramidze reported on the phase III EMPOWER-Lung 3 trial part 1 (NCT03409614), which evaluated the combination of cemiplimab plus ipilimumab and CT as first-line treatment of patients with advanced squamous or non-squamous NSCLC with PD-L1 expression < 50 % [16].
Eligible patients had a histologically or cytologically documented squamous or non-squamous NSCLC with stage IIIB or IIIC (not amenable to definitive concurrent chemoradiation) or stage IV (with no prior systemic treatment for recurrent or metastatic disease) disease, at least one radiographically measurable lesion per RECIST v1.1 criteria and an ECOG performance status of 0 or 1.
Patients were randomized (1:1:1) to receive either cemiplimab (350 mg, IV, Q3W for up to 108 weeks) plus four cycles of standard platinum-based doublet CT (CC Arm), or cemiplimab plus a reduced CT regimen (2 cycles) plus ipilimumab (50 mg, IV, Q6W for up to four cycles) (CIC Arm), or four cycles of standard platinum-based doublet CT (StC Arm). The primary endpoint was the OS, while the PFS, ORR, patient-reported outcomes (PROs) and safety were secondarily analyzed.
Of note, part 1 of this trial was stopped prematurely as patients were then reprioritized to standard CT plus cemiplimab regardless of PD-L1 expression. Thus, only descriptive statistical analyses comparing the combination of cemiplimab plus ipilimumab with a reduced course of CT (CIC Arm) with a standard CT regimen (StC Arm) were done and presented at the ESMO IO 2022 meeting [16].
A total of 323 patients, 85 % males, were enrolled in the study with a median followed-up of 35.5 months. Almost half of the patients (49 % in each group) presented with a PD-L1 expression < 1 %, while there was no patient with a PD-L1 expression > 50 %. Patients in the CIC arm (n = 109) showed a longer OS than those in the StC arm (n = 106) with a median OS of 20.1 months versus 13.9 months (HR = 0.615; 95 % CI, 0.441-0.857), respectively. The median PFS was 6.4 months versus 6.3 months (HR = 0.813; 95 % CI, 0.596-1.108), respectively. Overall, 35.8 % of patients in the CIC Arm responded to the treatment (ORR) compared to 28.3 % in the StC Arm. The median DoR was longer for CIC-treated patients compared
to those having received the standard CT regimen alone (15.9 months vs 6.3 months, respectively).
Safety outcomes were comparable to known historical safety data, with an incidence of grade ≥ 3 TEAEs of 43.1 % in the CIC Arm and 42.7 % in the StC Arm. TEAEs leading to treatment discontinuation occurred in 6 % versus 2 % of the patients, respectively, while the rate of TEAEs leading to death was 4.6 % versus 4.9 %, respectively.
Although only descriptive data were presented, this trial showed that the addition of cemiplimab plus ipilimumab to a reduced CT regimen prolonged the overall survival in patients with PD-L1 expression < 50 %.
REFERENCES
- Forde, PM, et al., Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022; 386(21): 1973-1985.
- Shu, CA, et al., Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. The Lancet Oncology 2020; 21(6): 786-795.
- Zhao, ZR, et al., Phase 2 trial of neoadjuvant toripalimab with chemotherapy for resectable stage III non-small-cell lung cancer. OncoImmunology 2021; 10(1): 1996000.
- Provencio, M, et al., Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. The Lancet Oncology 2020; 21(11): 1413-1422.
- Ren, S, et al., Camrelizumab Plus Carboplatin and Paclitaxel as First-Line Treatment for Advanced Squamous NSCLC (CameL-Sq): A Phase 3 Trial. Journal of Thoracic Oncology 2022; 17(4): 544-557.
- Zhou, C, et al., Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. The Lancet Respiratory Medicine 2021; 9(3): 305-314.
- Lei, J, et al., A randomized, controlled, multicenter phase II trial of camrelizumab combined with albumin-bound paclitaxel and cisplatin as neoadjuvant treatment in resectable stage IIIA and IIIB (T3N2) non-small-cell lung cancer. Ann Oncol 2022; 16 (Suppl. 1): Abstract 560.
- Zhang, L, et al., Tislelizumab: A Modified Anti-tumor Programmed Death Receptor 1 Antibody. Cancer Control 2022; 29: 10732748221111296.
- Wang, J, et al., Tislelizumab Plus Chemotherapy vs Chemotherapy Alone as First-line Treatment for Advanced Squamous Non–Small-Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncology 2021; 7(5): 709.
- Wang, J, et al., Randomized phase III study of tislelizumab plus chemotherapy versus chemotherapy alone as first-line treatment for advanced squamous non-small cell lung cancer (sq-NSCLC): RATIONALE-307 updated analysis. Ann Oncol 2022; 16 (Suppl. 1): Abstract 132P.
- Wang, J, et al., Tislelizumab Plus Chemotherapy vs Chemotherapy Alone as First-line Treatment for Advanced Squamous Non-Small-Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol 2021; 7(5): 709-717.
- Dhillon, S, et al., Penpulimab: First Approval. Drugs 2021; 81(18): 2159-2166.
- Han, B, et al., Final analysis of AK105-302: A randomized, double-blind, placebo-controlled, phase III trial of penpulimab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC. Ann Oncology 2022; 16 (Suppl. 1): Abstract 59MO.
- Villani, A, et al., Cemiplimab for the treatment of advanced cutaneous squamous cell carcinoma. Expert Opin Drug Saf 2022; 21(1): 21-29.
- Sezer, A, et al., Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. The Lancet 2021; 397(10274): 592-604.
- Baramidze, A, et al., Cemiplimab (cemi) + platinum doublet chemotherapy (chemo) + ipilimumab (ipi) for first-line treatment of advanced non-small cell lung cancer (NSCLC): EMPOWER-Lung 3 part I. Ann Oncol 2022; 16 (Suppl. 1): Abstract 122MO.
© 2023 Springer-Verlag GmbH, Impressum
More posts
Emerging therapies in solid tumors
Interleukin-8 (IL-8), also known as chemokine (C-X-C motif) ligand 8, is a pro-inflammatory chemokine that exerts direct pro-tumorigenic effects primarily by recruiting immunosuppressive cells into the tumor microenvironment such as neutrophils and myeloid-derived suppressor cells. IL-8 has also been shown to promote cancer progression and resistance to therapy, by inducing angiogenesis, epithelial-mesenchymal transition (EMT), and cancer stem cell (CSC) self-renewal.
New strategies with PD-1/PD-L1 blockade in lung cancer
Small-cell lung cancer (SCLC) accounts for about 15 % of all diagnosed cases of lung cancer and is characterized by a high proliferative rate, an early development of widespread metastases and a poor prognosis. The five-year survival rate is less than 7 %. More than two-thirds of patients with this highly aggressive neuroendocrine tumor are diagnosed with advanced or extensive-stage disease (ES-SCLC).
Preface – ESMO IO 2022
The ESMO Immuno-Oncology Congress took place in Geneva, Switzerland, and virtually from 7th to 9th December 2022. In total, more than 2,000 participants from more than 100 countries attended one of the 31 sessions featuring over 249 presented abstracts, 6 late breaking abstracts, 6 proffered paper, 14 mini orals and 229 posters.