New strategies with PD-1/PD-L1 blockade in lung cancer
Small-cell lung cancer (SCLC) accounts for about 15 % of all diagnosed cases of lung cancer and is characterized by a high proliferative rate, an early development of widespread metastases and a poor prognosis [1]. The five-year survival rate is less than 7 % [2]. More than two-thirds of patients with this highly aggressive neuroendocrine tumor are diagnosed with advanced or extensive-stage disease (ES-SCLC) [3]. For more than two decades, the standard upfront treatment was platinum-based chemotherapy (CT), associated with a median survival time of less than one year [4]. Recently, the addition of immune checkpoint inhibitors (ICIs) targeting the programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) to standard CT have demonstrated sustained OS benefit and have rapidly emerged as the current standard-of-care in the first-line setting [5]. Despite this progress, resistances emerge in virtually all patients; therefore, ES-SCLC remains a therapeutically challenging disease.
AdvanTIG-105 trial: 1L ociperlimab plus tislelizumab plus CT
T-cell immunoglobulin and immunoreceptor tyrosine–based inhibitory motif (ITIM) domain (TIGIT) is an coinhibitory receptor primarily expressed on activated T-cells, Tregs and natural killer (NK) cells (7). It is a promising target in the field of ES-SCLC immunotherapy. Combining TIGIT inhibitors with programmed PD-1/PD-L1 inhibitors has previously demonstrated antitumor activity in advanced solid tumors [6, 7]. Ociperlimab is a novel humanized Fc-intact IgG1 monoclonal antibody (mAb) designed to bind to TIGIT with a high specificity and affinity [8] whereas tislelizumab is an anti-PD-1 mAb with low Fcγ binding affinity on macrophages [9].
At this year’s ESMO IO meeting, Jun Zhang reported on the dose-expansion study of the ongoing phase 1b AdvanTIG-105 trial (NCT04047862) investigating the combination of ociperlimab plus tislelizumab and chemotherapy (CT) in patients with ES-SCLC [10]. A preliminary dose-escalation study (phase I) has previously demonstrated the antitumor activity and tolerability of this combination in patients with advanced solid tumors [11].
Patients included in the dose-expansion phase (Cohort 4) had a confirmed ES-SCLC, no prior systemic treatment for metastatic disease and an ECOG scorings of 0 or 1. They first received four cycles of the recommended phase II dose (RP2D) of ociperlimab (900 mg, intravenously [IV] every three weeks [Q3W]) plus tislelizumab (200 mg, IV, Q3W) and CT (cisplatin [75 mg/m2] or carboplatin, area under the curve [AUC] 5, on Day 1 plus etoposide [100 mg/m2 on Days 1 to 3]). After four cycles, patients were administered with the RP2D of ociperlimab and tislelizumab until disease progression (PD) or intolerable toxicity. The primary endpoint was the overall response rate (ORR) as assessed by the investigator per RECIST v1.1. Key secondary endpoints included the investigator-assessed progression-free survival (PFS), the duration of response (DoR), the disease control rate (DCR) per RECIST v1.1, and safety.
Out of 42 patients enrolled in Cohort 4 (data cut-off date of June 20, 2022), 40 were evaluable for efficacy, as defined by 1 or more evaluable post-baseline tumor response assessment. The median age was 65.5 years, and most patients were male (76.2 %). The median follow-up was 24.9 weeks (range, 3.0-67.9).
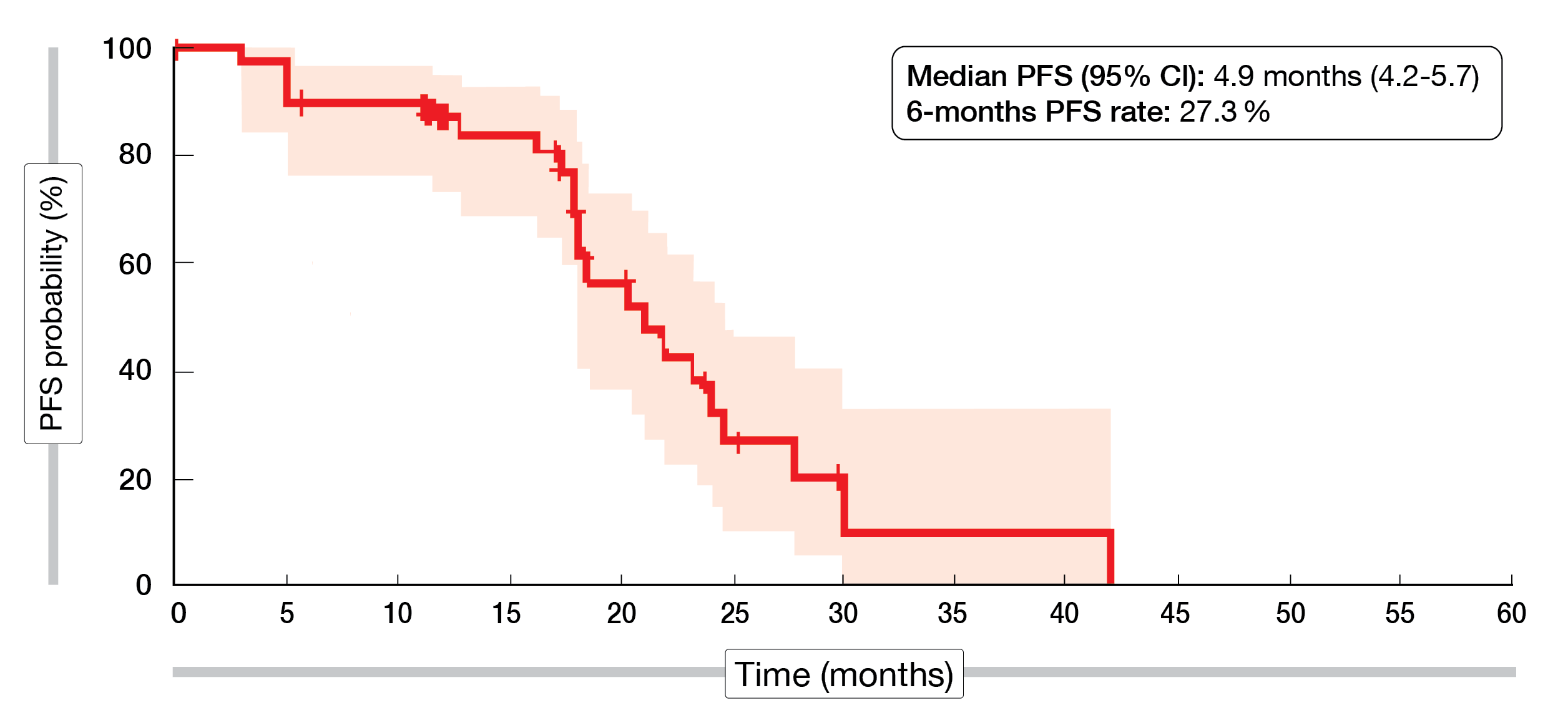
In total, 65 % of the patients achieved a confirmed ORR (95 % CI, 48.3-79.4; partial response [PR], 65 %; complete response [CR], 0 %), while 25 % of the cohort had a stable disease (SD) and 5 % a progressive disease (PD). The median DoR was 4.3 months (95 % CI, 3.2-5.6), the median PFS 4.9 months (95 % CI, 4.2-5.7), while the 6-month PFS rate reached 27.3 % (Figure 1).
Grade ≥ 3 treatment-emergent adverse events (TEAEs) occurred in 59.5 % of the patients, of these, decreased neutrophil count (33.3 %) and white blood cell count (16.7 %) were the most frequently reported ones. Overall, 40.5 % of the patients had serious TEAEs and 28.6 % experienced immune-mediated TEAEs, including two patients with grade ≥ 3. Moreover, two patients died following AEs (one of pneumonia unrelated to treatment and one of cardiac arrest). In total, 4.8 % of the AEs led to ociperlimab discontinuation.
The authors concluded that the combination of ociperlimab plus tislelizumab with cisplatin or carboplatin plus etoposide showed encouraging antitumor activity as first-line treatment in patients with ES-SCLC accompanied by an acceptable safety profile.
Figure 1: AdvanTIG-105 trial – Kaplan-Meier curve of the progression-free survival of patients treated with ociperlimab and tislelizumab plus chemotherapy.
2L COM701 ± nivolumab
Several agents, such as the novel 1st-in-class immune checkpoint inhibitor (ICI) COM701, are currently under investigation in a post-ICI second line setting. COM701 is a humanized IgG4 monoclonal antibody with a high affinity to poliovirus receptor related immunoglobulin domain containing (PVRIG) [12]. PVRIG blockade leads to enhanced activation of T-cells and NK cells. Historical data with Lung-MAP demonstrated the superiority of combining immunotherapeutic agents (ramucirumab – an anti-vascular endothelial growth factor (VEGF) antibody and pembrolizumab – an anti-PD-1 ICI) over the standard-of-care, with a median OS of 14.5 months vs 11.6 months, respectively [13].
Sullivan et al. evaluated the antitumor activity of COM701 with or without nivolumab in an ongoing phase I trial (NCT03667716) conducted in patients with metastatic NSCLC who have received prior PD-1/PD-L1 inhibitor(s) [14]. Preliminary safety and tolerability outcomes have previously been reported at ASCO 2021 meeting [12]. At this year’s ESMO IO conference, Ryan Sullivan presented long-term follow-up results.
Key inclusion criteria were a histologically confirmed locally advanced or metastatic solid malignancy with no available standard treatment, an ECOG of 0 or 1, and one or more prior lines of PD-1/PD-L1 inhibitor therapy. Patients with an active autoimmune disease requiring a systemic treatment, or a prior anti-PVRIG treatment or a history of immune-related toxicities leading to treatment discontinuation were excluded from the study. Patients received COM701 with or without nivolumab at variable doses. The co-primary endpoints were safety and tolerability of the mono- or combined therapy, while anti-tumor activity was set as a secondary endpoint. Other exploratory outcomes were COM701 immunogenicity, pharmacodynamics effect in blood, as well as cytokines and immunophenotype variations.
A total of seven patients were enrolled at the time of data cut-off (July 17, 2022). Out of five patients receiving COM701 monotherapy, one took part in the dose escalation phase at a dose of 0.01 mg/kg (IV, Q3W), while four were enrolled during the dose expansion phase at a dose of 20 mg/kg (IV, Q4W) which was the recommended dose for expansion. Two patients received the combination therapy during the dose escalation phase: one received COM701 (3 mg/kg, IV, Q3W) plus nivolumab (360 mg, IV, Q3W) and one received COM701 (10 mg/kg, IV, Q4W) plus nivolumab (480 mg, IV, Q4W).
Four patients (57 %) were over 65 years old and most of them were female (n = 6). The median number of prior lines of therapy was four (range, 3-6). All patients had received at least one prior line of ICI, and four of them (57 %) were administered more than two prior ICI lines. Overall, 71 % of the patients had a controlled disease (CR = 0 %, PR = 0 %, SD = 71 %), whereas 29 % had a progressive disease. For two patients with SD, the response was prolonged over six months. The median PFS in all patients was 84 days (95 % CI, 22-231) and was longer with the combination therapy than compared with the monotherapy (5.72 months [95 % CI, 3.15-NA] vs 2.69 months [95 % CI, 1.71-NA]). The median OS was 9.5 months (95 % CI, 2.7-11), with a median OS of 9.53 months (95 % CI, 4.11-NA) in the monotherapy group and 10.10 months (95 % CI, 8.57-NA) in the combination group.
The combination of COM701 plus nivolumab for the treatment of NSCLC patients post ICI showed promising results in terms of antitumor activity. A phase I trial in NSCLC patients post-ICI treatment evaluating COM902 plus COM701 with a PD-1 inhibitor versus COM902 plus COM701 with chemotherapy is planned.
Neoadjuvant SHR-1701 in unresectable NSCLC
Consolidation immunotherapy after chemoradiation is the actual standard-of-care for stage III unresectable NSCLC (uNSCLC) [15]. In patients with resectable NSCLC, neoadjuvant immunotherapy combined with CT has already proved to elicit strong tumor-specific T-cell response as well as to induce tumor and nodal downstaging [16-18]. At this year’s ESMO IO meeting Yi-Long Wu presented the preliminary data of a phase II trial (NCT04580498), conducted in uNSCLC stage III naïve patients, evaluating the combination of a neoadjuvant immunotherapy with a PD-L1 expression-dependent CT, followed by surgery or radiotherapy (RT) [19]. Patients received a novel bifunctional fusion protein, SHR-1701, targeting both PD-L1 and transforming growth factor beta (TGF-β). This promising molecule has previously been evaluated in a phase I trial (NCT03710265) and demonstrated an encouraging antitumor activity, as well as an acceptable tolerability in advanced solid tumors [20].
This multicenter, open-label phase II trial included patients with unresectable stage III NSCLC, with an ECOG performance status of 0 or 1, no prior systemic therapy nor radiotherapy of the thorax, and no sensitizing EGFR/ALK alterations. Patients with a PD-L1 tumor proportional score (TPS) < 50 % received SHR-1701 (30 mg/kg, Day 1) plus paclitaxel (175 mg/m2, Day 1) plus carboplatin (AUC 5, Day 1), Q3W for three cycles (Arm A). Patients with a PD-L1 TPS score ≥ 50 % were randomized 1:1 to receive either SHR-1701 (30 mg/kg, Day 1) plus paclitaxel (175 mg/m2, Day 1) plus carboplatin (AUC 5, Day 1), Q3W for three cycles (Arm B) or SHR-1701 (30 mg/kg, Day 1) Q3W for three cycles (Arm C). Following multidisciplinary consultation, patients of the three arms allocated to either definitive surgery or definitive RT (60 Gy/30 fractions) plus concurrent cisplatin (30 mg/m2, QW), followed by SHR-1701 (30 mg/kg), Q3W for 16 cycles or until PD or unacceptable toxicity. The co-primary endpoints were post-induction ORR and event-free survival (EFS), while secondary endpoints included OS, time to distant metastasis (TTDM) and safety. Other key exploratory endpoints were the major pathological response (MPR) and the surgical rate.
At the data cut-off date (July 31, 2022), a total of 107 patients were enrolled in the study. Out of them, 82.2 % had a PD-L1 TPS < 50 % (Arm A) and 17.8 % a PD-L1 TPS ≥ 50 % (Arm B, n = 9 or Arm C, n = 10). The median age was about 59 years. Overall, 73.8 % presented with NSCLC of squamous histology.
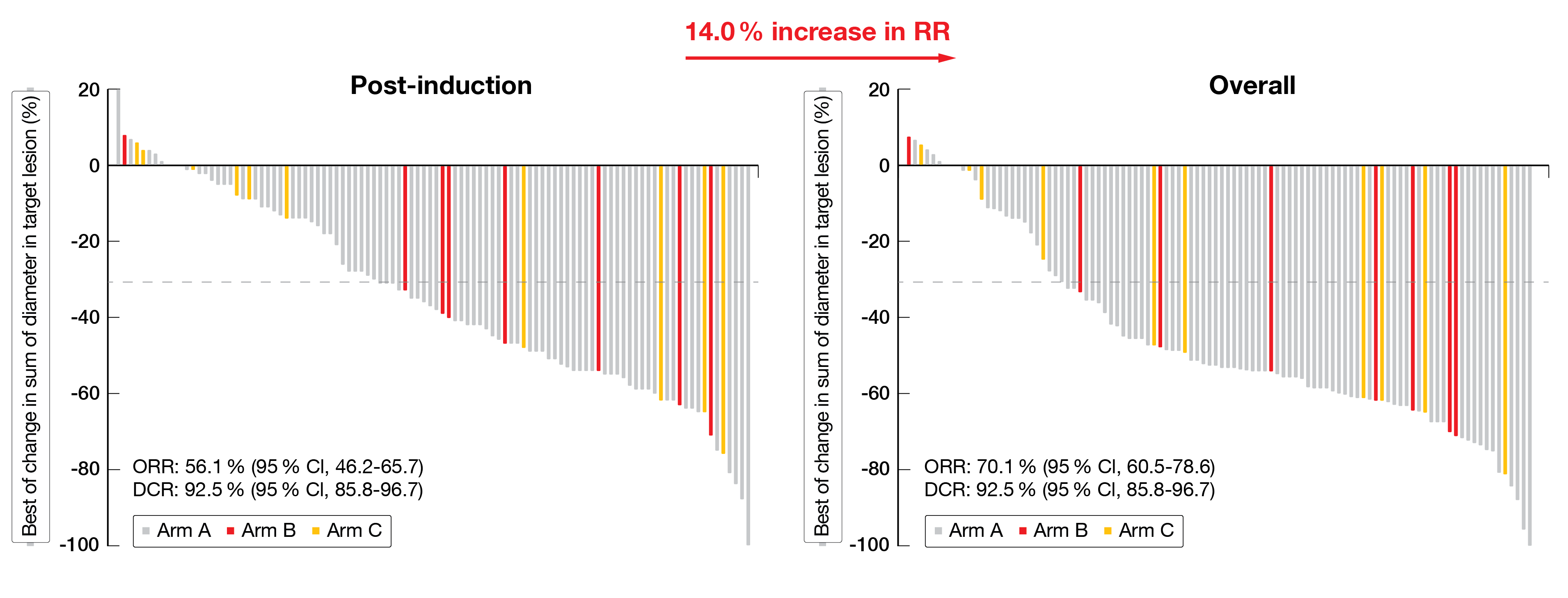
Post-induction ORR was 56.1 % (95 % CI, 46.2-65.7) in all patients (Arms A + B + C). Patients responded better to a combination therapy (Arms A + B) than to SHR-1701 monotherapy (Arm C); median ORR, 57.7 % in Arms A + B vs 40.0 % in Arm C, respectively. The median DCR was 92.5 % (95 % CI, 85.8-96.7) in all patients (Arms A + B + C). In more details, the post-induction CR, PR and SD rates of Arms A + B versus Arm C accounted for 1.1 %, 56.7 % and 35.1 % versus 0 %, 40.0 % and 50.0 %, respectively. The ORR based on assessments throughout the treatment course including RT-treated patients accounted for 71.1 % in Arms A +B compared to 60.0 % in Arm C (Figure 2).
The overall median EFS was 18.2 months (95 % CI, 11.8-NR), 14.9 months in the combination therapy arm (Arms A + B), and not yet reached in the monotherapy arm. A total of 27 patients (25.2 %) had surgery (Arm A, n = 77.8 %; Arm B, n = 11.1 %; Arm C, n = 11.1 %), of which ten patients had a stage IIIA disease, twelve a stage IIIB disease and five a stage IIIC disease at enrollment. In total, 74.1 % of patients had had a post-induction radiographic PR and the rest a stable disease. About two-third of the patients showed a pathological nodal downstaging. The MPR rate (defined as ≤ 10 % residual viable tumor cells in the resected primary tumor) was 44.4 % and the pCR rate (defined as no residual viable tumor cells in the resected primary tumor and lymph nodes) was 25.9 %. The EFS was not reached (95 % CI, 11,8-NR) in resected patients and 14.9 months (95 % CI, 11.4-NR) in RT-treated patients. Moreover, the 12-month EFS rate was 74.4 % in resected patients compared to 55.9 % in patients who received definitive RT.
In total, 72 % experienced grade ≥ 3 TRAEs, including a decrease in neutrophil, white blood cell and lymphocyte counts as the most frequent ones. The rate of TRAEs leading to SR-1701 discontinuation was 15.9 % and the rate of immune-related AEs was 57.0 %. Three patients died in Arms A + B.
The neoadjuvant administration of SHR-1701 with or without CT followed by surgery or RT induced a promising antitumor activity with an acceptable safety in patients with uNSCLC. Importantly, this neoadjuvant regimen rendered 25.2 % of unresectable patients eligible for surgery and resulted in a favorable efficacy in resected patients.
Figure 2: Radiographic assessment of best change in lesion tumor size after neoadjuvant SHR-1701 in patients with unresectable NSCLC.
1L HDACi + tislelizumab + CT
The regulation of histone acetylation, which is an important epigenetic regulation mechanism controlled by histone acetyltransferases (HATs) and histone deacetylases (HDACs), is associated with resistance to immunotherapy. Aberrant expression of HDACs have frequently been observed in various human cancers. HDAC inhibitors (HDACis) have displayed a therapeutic potential in treating cancer and are now emerging agents in cancer therapy [21]. Moreover, HDACis have been suggested to potentially synergize with PD-1 antibodies by inducing and activating the activation of the NK and cytotoxic T-cell – mediated cellular immunity, thus probably resulting in a delayed or prevented resistance to ICI [22]. At the ESMO IO 2022 meeting, Lijie Wang reported data of a phase II trial (chiCTR2000041542) evaluating the antitumor activity and tolerability of combed chidamide (a subtype-selective HDACi) and with tislelizumab (an anti-PD-1 monoclonal antibody approved for the first line treatment of NSCLC) plus chemotherapy [23].
To participate in this open-label, non-randomized study, patients were required to have a histologically confirmed squamous or non-squamous NSCLC of clinical stage IIIB to IV, no prior systemic treatment and an ECOG performance status of 0 or 1. Exclusion criteria were an EGFR/ALK mutation or fusion and symptomatic brain metastases. Patients received chidamide (20 mg, twice a week [BIW], per os [PO]), tislelizumab (200 mg, Q3W, IV) and CT (Q3W) for four to six cycles. The maintenance regimen consisted of chidamide (20 mg, BIW, PO) and tislelizumab (200 mg, Q3W, IV) until progression, unacceptable toxicity, withdrawal of consent or death. The ORR was set as the primary outcome, while DCR, PFS and safety were secondarily analyzed. Tumor response was assessed per RECIST v1.1.
Among the 20 patients included in this study, the median age was 63.5 (range, 49-75) years. All patients were male, and only 10 % of them were never-smokers. Ten patients (50 %) presented with an adenocarcinoma and ten (50 %) with a squamous cell carcinoma. Most of them (95 %) had a clinical stage of IV and 40 % had a PD-L1 status ≥ 1 %. At the time of data cut-off (September 15, 2022), 70 % of the patients were still on study therapy, and 19 patients were evaluable for efficacy. The ORR was 73.7 % (95 % CI, 63.6-83.8), including one patient (5.3 %) with a CR and 13 patients (68.4 %) with a PR. Of note, the ORR was higher in patients with a squamous cell carcinoma histology than in those with adenocarcinoma (88.9 % vs 60 %). All study participants reached a disease control (DCR = 100 %). After a median follow-up of 10.7 months, the median PFS was 13.8 months (95 % CI, 5.4-22.2) and the 1-year PFS rate reached 76.6 % (95 % CI, 64.3- 88.9).
Overall, grade ≥ 3 TRAEs were detected in 55 %, the most frequent ones being leukopenia (25 %), neutropenia (20 %) and thrombocytopenia (15 %). Immunotherapy discontinuation occurred in 20 % of the patients, followed by pneumonia (10.0 %), diarrhea (5.0 %), or dermatitis (5.0 %).
This preliminary analysis demonstrated an encouraging antitumor activity of HDACi combined with anti-PD1 antibody and CT in the 1L setting of advanced NSCLC. The safety profile was favorable and updated follow-up data are expected shortly.
KRYSTAL-1 and -7 trials: adagrasib plus pembrolizumab
About 14 % of patients with NSCLC harbor KRASG12C mutations. Adagrasib, a covalent KRASG12C inhibitor, has a half-life of 23-hours, dose-dependent pharmacokinetics, and the capability to penetrate the central nervous system. In the phase I/Ib part of the KRYSTAL-1 study, adagrasib already showed a favorable clinical activity accompanied by an acceptable tolerability in patients with KRASG12C-mutant cancers including NSCLC [24]. Several preclinical data support combining adagrasib with immunotherapy as a potential antitumor strategy. In this context, adagrasib administration has been shown to reverse an immunosuppressive tumor microenvironment and to induce tumor sensitization to ICI in mice [25]. KRYSTAL-1 (NCT03785249) phase Ib and KRYSTAL-7 (NCT04613596) phase II trials evaluates the safety and efficacy of adagrasib plus pembrolizumab in treatment naïve (TN) patients harboring a KRASG12C mutation. Preliminary study data were presented at this ESMO IO 2022 by Pasi A. Jänne [26].
Patients’ eligibility criteria include advanced, unresectable, or metastatic NSCLC with KRASG12C mutation, and no prior systemic therapy for locally advanced or metastatic disease. Stable brain metastases are tolerated. In the KRYSTAL-1 phase Ib trial, the primary endpoint is safety, while the secondary endpoints enclose the ORR as assessed per RECIST v1.1, DoR, PFS and OS. In the KRYSTAL-2 phase II trial, patients with a PD-L1 TPS < 1 % are allocated to Cohort 1a, whereas patients with a PD-L1 TPS ≥ 1 % are allocated to Cohort 2. The primary outcome is the ORR as assessed by RECIST v1.1, while DoR, PFS, OS, safety and PK are set as secondary outcomes. Both cohorts receive adagrasib (400 mg, BID) and pembrolizumab (200 mg, IV, Q3W).
At the time of data cut-off (August 30, 2022), seven patients had already been enrolled in the KRYSTAL-1 trial and 75 in KRYSTAL-7 (Cohort 1a, n = 11; Cohort 2, n = 64). Among KRYSTAL-1 participants, four out of seven patients attained an objective response (ORR = 57 %) and the disease control rate (DCR) was 100 %. Responses occurred in 2/2 patients with PD-L1 TPS ≥ 50 %, 1/4 patients with PD-L1 TPS 1–49 %, and 1/1 patient with PD-L1 TPS < 1 %. All four responding patients had a DoR of at least nine months and two still receiving treatment beyond 18 months. Overall, four patients experienced grade 3 TRAEs, whereas no patient had grade 4 or 5 TRAEs.
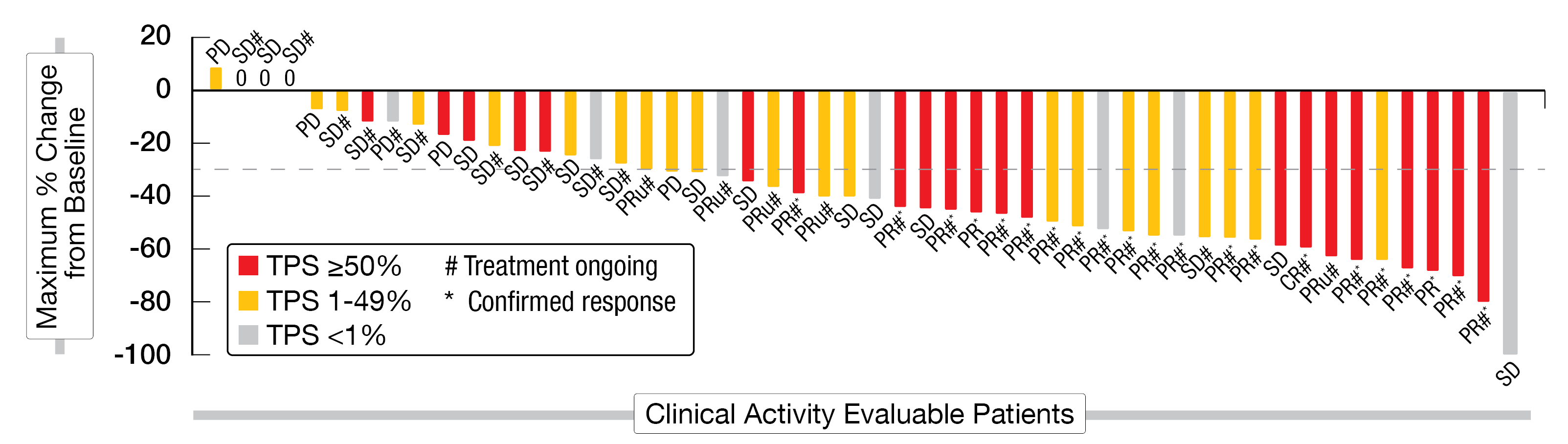
Patients of the KRYSTAL-7 trial were followed for a median duration of 3.5 months and were treated for a median time of two months. Out of 75 enrolled patients, 53 were clinically evaluable (≥ 1 post-baseline tumor assessment) and 25 were enrolled for at least six months before the data cut-off date. The median age of the enrolled patients was 66 years (range, 40-84). In total, 1 % of them were never smokers and 15 % had a PD-L1 TPS < 1 %. The ORR was 49 % (95 % CI, 35-63) and the DCR 89 % (CR, 2 %; PR, 47 %; SD, 40 %) (Figure 3). Responses were observed in 59 % of patients with PD-L1 TPS ≥ 50 %, 48 % with PD-L1 TPS 1–49 %, and 30 % with PD-L1 TPS < 1 %, respectively. The median time to response was 1.4 months. Out of the 26 responding patients, six had a delayed response (> 2 months). So far, 35 of 53 patients, including all responding patients, were still on treatment at the time this analysis was performed.
Grade 3 TRAEs occurred in 40 % of patients with increased aspartate aminotransferase (AST, 9 %) or alanine aminotransferase (ALT, 8 %) being the most frequent ones. TRAEs led to adagrasib dose reduction in 31 %, dose interruption in 41 %, discontinuation of both drugs in 3 % and of pembrolizumab in 3 %. Grade 4 TRAES (4 %) comprised one case each of pneumonitis, neutropenia, and pulmonary embolism. No grade 5 TRAEs were observed.
According to those preliminary data, adagrasib plus pembrolizumab induced a clinically meaningful antitumor activity across all PD-L1 subgroups in patients KRASG12C-mutated NSCLC. The safety profile was manageable and similar to that observed of each agent as monotherapy. Further phase III trials comparing this combination versus standard-of-care in this patient population are planned.
Figure 3: Best change in tumor size after adagrasib plus pembrolizumab in KRASG12C mutated NSCLC (KRYSTAL-7 trial).
REFERENCES
- Rudin, CM, et al., Small-cell lung cancer. Nature Reviews Disease Primers 2021; 7(1): 3.
- Wang, S, et al., Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Scientific Reports 2017; 7(1): 1339.
- Oronsky, B, et al., What’s New in SCLC? A Review. Neoplasia 2017; 19(10): 842-847.
- Oronsky, B, et al., A 2022 Update on Extensive Stage Small-Cell Lung Cancer (SCLC). J Cancer 2022; 13(9): 2945-2953.
- Horn, L, et al., First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. NEJM 2018; 379(23): 2220-2229.
- Niu, J, et al., 410 P Safety and efficacy of vibostolimab, an anti-TIGIT antibody, plus pembrolizumab in patients with anti-PD-1/PD-L1-naive NSCLC. J Clin Oncol 2020; 31(S4):S891-892.
- Rodriguez-Abreu, D, Primary analysis of a randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1L) treatment in patients with PD-L1-selected NSCLC (CITYSCAPE). J Clin Oncol 2020; 38: Abstract 9503.
- Chen, X, et al., An Fc-Competent Anti-Human TIGIT Blocking Antibody Ociperlimab (BGB-A1217) Elicits Strong Immune Responses and Potent Anti-Tumor Efficacy in Pre-Clinical Models. Frontiers in Immunology 2022; 13: 828319.
- Zhang, T, et al., The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunology, Immunotherapy 2018; 67(7): 1079-1090.
- Zhang, J, et al., AdvanTIG 105: Phase 1b Dose Expansion Study of Ociperlimab Plus Tislelizumab With Chemotherapy in Patients With Extensive Stage Small Cell Lung Cancer. Ann Oncol 2022; 33 (Suppl 7): Abstract 148P.
- Frentzas, S, et al., AdvanTIG-105: Phase 1 dose-escalation study of anti-TIGIT monoclonal antibody ociperlimab (BGB-A1217) in combination with tislelizumab in patients with advanced solid tumors. J Clin Oncol 2021; 39(S15): Abstract 2583.
- Vaena, DA, COM701 with or without nivolumab: Results of an ongoing phase 1 study of safety, tolerability and preliminary antitumor activity in patients with advanced solid malignancies (NCT03667716). J Clin Oncol 2021; 39(15): Abstract 2504.
- Reckamp, KL, et al., Phase II Randomized Study of Ramucirumab and Pembrolizumab Versus Standard of Care in Advanced Non–Small-Cell Lung Cancer Previously Treated With Immunotherapy—Lung-MAP S1800A. J Clin Oncol 2022; 40(21): 2295-2307.
- Sullivan, R, et al., COM701 ± nivolumab – preliminary results of antitumor activity from a phase 1 study in patients with metastatic NSCLC who have received prior PD-1/PD-L1 inhibitor. Ann Oncol 2022; 16 (Suppl. 1): Abstract 130P.
- Antonia, SJ, et al., Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. NEJM 2017; 377(20): 1919-1929.
- Chaft, JE, et al., Neoadjuvant atezolizumab for resectable non-small cell lung cancer: an open-label, single-arm phase II trial. Nature Medicine 2022; 28(10): 2155-2161.
- Forde, PM, et al., Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. NEJM 2022; 386(21): 1973-1985.
- Provencio, M, et al., Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. The Lancet Oncology 2020; 21(11): 1413-1422.
- Wu, YL, et al., A phase 2 study of neoadjuvant SHR-1701 with or without chemotherapy (chemo) followed by surgery or radiotherapy (RT) in stage III unresectable NSCLC (uNSCLC). Ann Oncol 2022; 16 (Suppl 1): Abstract LBA5.
- Liu, D, et al., Bifunctional anti-PD-L1/TGF-βRII agent SHR-1701 in advanced solid tumors: a dose-escalation, dose-expansion, and clinical-expansion phase 1 trial. BMC Medicine 2022; 20(1): 408.
- Li, Y, et al., HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harbor Perspectives in Medicine 2016; 6(10): a026831.
- Fischer, J, et al., Successful drug discovery, ed. W.E. Childers. Vol. 2. 2017: Weinheim: Wiley-VCH Verlag GmbH & Co. 270.
- Wang, L, et al., First-line HDACi plus Tislelizumab combined with chemotherapy in Advanced NSCLC. Ann Oncol 2022; 16 (Suppl. 1): Abstract 131P.
- Jänne, PA, et al., Adagrasib in Non–Small-Cell Lung Cancer Harboring a KRASG12C Mutation. NEJM 2022; 387(2): 120-131.
- Briere, DM, et al., The KRASG12C Inhibitor MRTX849 Reconditions the Tumor Immune Microenvironment and Sensitizes Tumors to Checkpoint Inhibitor Therapy. Mol Cancer Ther 2021; 20(6): 975-985.
- Jänne, PA, Preliminary safety and efficacy of adagrasib with pembrolizumab in treatment-naïve patients with advanced non-small cell lung cancer (NSCLC) harboring a KRASG12C mutation. Ann Oncol 2022; 16 (Suppl. 1): Abstract LBA4.
© 2023 Springer-Verlag GmbH, Impressum
More posts
Emerging therapies in solid tumors
Interleukin-8 (IL-8), also known as chemokine (C-X-C motif) ligand 8, is a pro-inflammatory chemokine that exerts direct pro-tumorigenic effects primarily by recruiting immunosuppressive cells into the tumor microenvironment such as neutrophils and myeloid-derived suppressor cells. IL-8 has also been shown to promote cancer progression and resistance to therapy, by inducing angiogenesis, epithelial-mesenchymal transition (EMT), and cancer stem cell (CSC) self-renewal.
New strategies with PD-1/PD-L1 blockade in lung cancer
Small-cell lung cancer (SCLC) accounts for about 15 % of all diagnosed cases of lung cancer and is characterized by a high proliferative rate, an early development of widespread metastases and a poor prognosis. The five-year survival rate is less than 7 %. More than two-thirds of patients with this highly aggressive neuroendocrine tumor are diagnosed with advanced or extensive-stage disease (ES-SCLC).
Preface – ESMO IO 2022
The ESMO Immuno-Oncology Congress took place in Geneva, Switzerland, and virtually from 7th to 9th December 2022. In total, more than 2,000 participants from more than 100 countries attended one of the 31 sessions featuring over 249 presented abstracts, 6 late breaking abstracts, 6 proffered paper, 14 mini orals and 229 posters.