Checkpoint inhibition excels in all treatment lines
Neoadjuvant therapy: NEOSTAR
Patients with early and locally advanced (stage I-IIIA) non–small-cell lung cancer (NSCLC) usually undergo surgery, but long-term outcomes leave much to be desired. After surgery alone, the recurrence rate is substantial at more than 50 % [1]. Perioperative chemotherapy as a means to prevent disease recurrence only confers a 5 % improvement in 5-year survival compared to sole surgery [2, 3]. Based on these observations, anti-PD-1 therapy is being tested in the neoadjuvant setting with the goal of priming a specific anti-tumour response and eradicating micrometastases [4].
In the open-label, randomised, phase II NEOSTAR trial, 36 patients with stage I-IIIA NSCLC who were amenable to resection received either nivolumab monotherapy 3 mg/kg for 3 doses (Arm A) or nivolumab 3 mg/kg for 3 doses plus ipilimumab 1 mg/kg for 1 dose (Arm B) prior to surgery [5]. Major pathological response (MPR; i.e., ≤ 10 % viable tumour cells) in both arms was defined as the primary endpoint. MPR is used as a surrogate for survival after neoadjuvant therapy. It was assumed that induction nivolumab and/or nivolumab plus ipilimumab would produce an MPR rate of at least 40 %, which exceeds the rate achieved with induction platinum-based chemotherapy. The study was not powered for an MPR comparison across the treatment arms.
Biomarker findings confirm activity
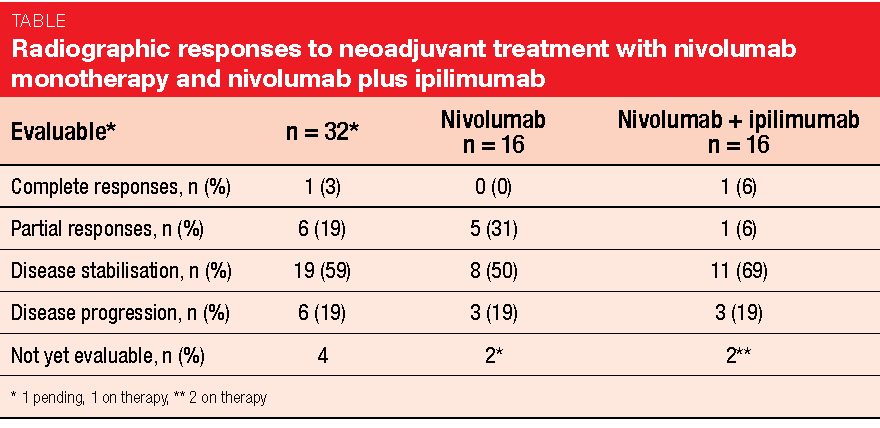
Neoadjuvant therapy was completed by 89 % of patients, and 84 % underwent surgery. In the resected group, the MPR rate was 31 %. With nivolumab and nivolumab plus ipilimumab, 28 % and 33 % of patients, respectively, achieved MPR. Nineteen percent of the patients in the resected group showed no viable tumour cells in their specimens (14 % and 25 % with nivolumab alone and the combination, respectively). Objective radiographic responses occurred in 22 % (31 % and 12 %, respectively; Table). A positive association was observed between radiographic responses and MPR (p < 0.002). Overall, neoadjuvant treatment with nivolumab and nivolumab plus ipilimumab was well tolerated.
According to biomarker analyses, both regimens significantly increased the percentages of proliferative and activated effector tumour-infiltrating lymphocytes (TILs) compared to untreated lung tumours. Moreover, compared to uninvolved lungs, the treatment increased T-cell receptor diversity in the tumours (p = 0.021). The combination appeared to induce greater proliferation of different T cell subsets than nivolumab alone, although the differences were not significant for CD8-positive TILs and CD4-positive regulatory T cells. Nivolumab plus ipilimumab was also shown to increase T-cell receptor homology in tumours compared to the uninvolved adjacent lung tissue (p = 0.048).
In their conclusion, the authors noted that this study adds to the growing neoadjuvant monotherapy data set and expands the neoadjuvant experience with a combination strategy. Limitations result from the small sample size in each arm. Exploratory biomarker analyses are ongoing.
Post-hoc analyses of PACIFIC
The phase III PACIFIC trial has established durvalumab as a standard of care in patients with unresectable, stage III NSCLC who had not experienced progression after definitive chemoradiotherapy. They were randomised to receive either durvalumab 10 mg/kg every 2 weeks (Q2W) for up to 12 months (n = 476), or placebo (n = 237). Significant benefits have been observed with the active treatment for both progression-free survival (PFS; 16.8 vs. 5.6 months; HR, 0.52; p < 0.001) [6] and overall survival (OS; not reached vs. 28.7 months; HR, 0.68; p = 0.0025) [7]. PACIFIC has been designed to evaluate durvalumab in all-comers, with PD-L1 testing not being mandatory. The PD-L1 status was unknown for 37 % of patients. Based on this trial, durvalumab has been globally approved for an all-comers population, including in the US and Japan, with the exception of the European Union where approval is limited to patients whose tumour cells express PD-L1 ≥ 1 %.
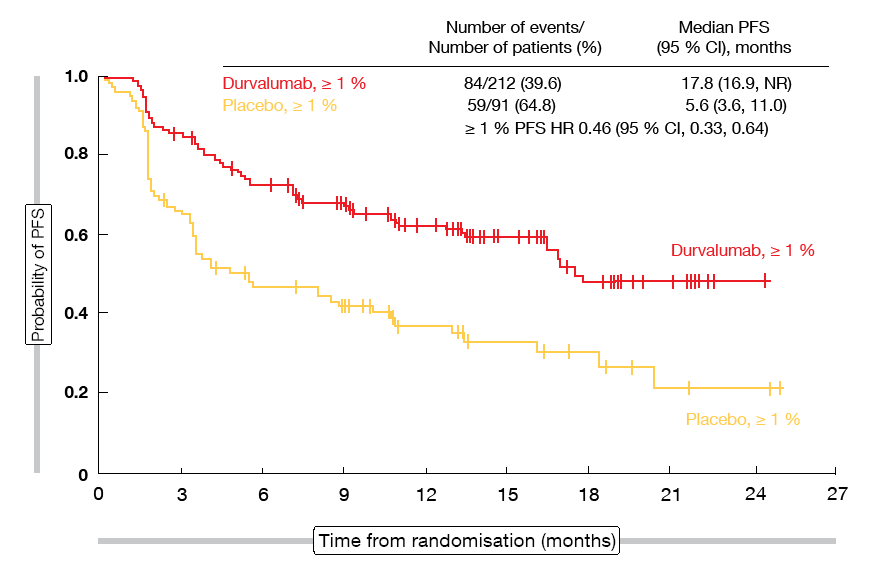
A post-hoc analysis reported at the ESMO 2018 Congress investigated outcomes in PACIFIC based on PD-L1 expression on one hand and components of the preceding concurrent chemoradiation on the other [8]. The PD-L1 analyses demonstrated that in patients whose tumour cells showed a PD-L1 expression ≥ 1 %, durvalumab gave rise to benefits with regard to PFS (17.8 vs. 5.6 months; HR, 0.46; Figure 1) and OS (not reached vs. 29.1 months; HR, 0.53). In those with PD-L1 expression < 1 %, PFS improvement was noted (10.7 vs. 5.6 months; HR, 0.73), whereas the results for OS were confounded by the performance of the placebo arm. In this group, the trajectory of the survival curves favoured durvalumab during the first 12 months, which corresponds to the time when the patients were on treatment, whereas the placebo-treated patients fared better during the remaining follow-up (HR, 1.36). Factors that might explain the over-performance of the placebo arm include the small number of events and the limited size of the subgroup, as well as imbalances in the baseline characteristics. Importantly, similar safety profiles were observed irrespective of PD-L1 expression. According to the authors, definite conclusions on outcomes by PD-L1 status cannot be drawn due to the limitations around the post-hoc exploratory subgroup analyses.
Furthermore, analyses tested the impact of the preceding treatments with respect to both chemotherapy and radiation dose. These data revealed consistent benefits of durvalumab regarding both PFS and OS irrespective of the type of chemotherapy, radiation dose used or the time from the end of radiation to randomisation. Likewise, toxicity profiles were similar regardless of the time from radiation. Overall, these data support the PACIFIC regimen of durvalumab following chemoradiation as the new standard of care in unresectable, stage III NSCLC.
Figure 1: Improvement in progression-free survival in the PD-L1 ≥ 1 % expression group treated with durvalumab in the PACIFIC trial
IMpower130: atezolizumab plus chemotherapy
First-line treatment of non-squamous stage IV NSCLC using atezolizumab as an add-on to chemotherapy was investigated in the randomised, open-label, phase III IMpower130 trial [9]. Carboplatin plus nab-paclitaxel constituted the chemotherapy backbone that was combined with atezolizumab in the experimental arm (n = 451) and administered alone in the control arm (n = 228). The findings support atezolizumab plus chemotherapy as a treatment option for advanced non-squamous NSCLC regardless of PD-L1 status. IMpower130 met its co-primary endpoints of PFS and OS in the intent-to treat wildtype population that comprised randomised patients excluding those with EGFR or ALK genomic alterations. The atezolizumab combination led to a 36 % reduction in the risk of progression and death (median PFS, 7.0 vs. 5.5 months; HR, 0.64; p < 0.0001). For mortality, this risk reduction amounted to 21 %, with a statistically significant and clinically relevant 4.7-month OS benefit (18.6 vs. 13.9 months; HR, 0.79 %; p = 0.033). PFS rates at 12 months were double with the atezolizumab-based treatment compared to the control arm (29.1 % vs. 14.1 %). Also, the analysis revealed a higher objective response rate (ORR) with the atezolizumab-based treatment (49.2 % vs. 31.9 %) and significantly prolonged median duration of response (8.4 vs. 6.1 months; p = 0.0004). At the time of the analysis, 36.8 % vs. 19.4 % of patients had ongoing responses.
Overall survival and PFS benefits occurred across all subgroups except for patients who had liver metastases at enrolment. Likewise, the addition of atezolizumab gave rise to PFS benefits across all PD-L1 cohorts (PD-L1–high, PD-L1–low, and PD-L1–negative). For OS, the results obtained in the PD-L1 subgroups favoured the experimental arm as well, although the differences between the treatment arms were not significant. The EGFR-/ALK-positive subgroup did not derive any statistically significant PFS or OS advantages. Atezolizumab plus chemotherapy had a safety profile consistent with the adverse events observed in the setting of single-agent therapy. The study yielded no new safety signals.
bTMB as a predictive marker
Tumour mutational burden (TMB) is an emerging predictive marker for checkpoint inhibitor therapy. However, adequate tumour tissue for TMB testing cannot always be obtained at diagnosis. Blood-based TMB (bTMB) therefore constitutes a non-invasive alternative that has recently been under evaluation. Kim et al. reported the primary efficacy results from the single-arm phase II B-F1RST study, which was the first prospective trial to test bTMB as a predictive biomarker for atezolizumab monotherapy in first-line NSCLC [10]. PD-L1–unselected patients with stage IIIB/IVA NSCLC of any histology (n = 152) received atezolizumab 1,200 mg Q3W until progression. The prespecified bTMB cut-off was defined at a score of 16. A total of 119 patients made up the biomarker-evaluable population (BEP), i.e. those with baseline evaluable blood samples showing adequate tumour content for testing. Ninety-one and 28 patients had low bTMB (< 16) and high bTMB (≥ 16), respectively.
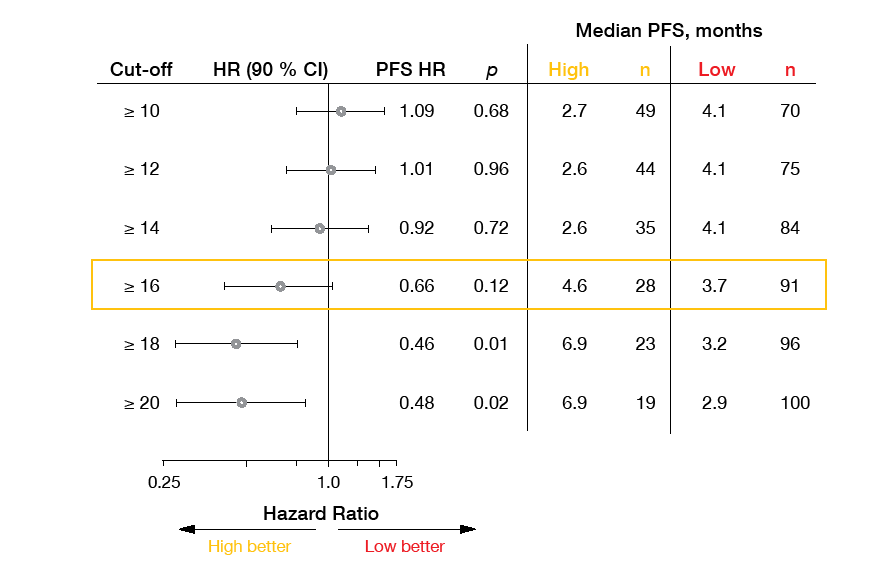
Investigator-assessed ORR, which was the efficacy endpoint, added up to 10.1 % in the BEP, with patients in the high bTMB subgroup showing significantly improved response rate compared with those in the low bTMB group (28.6 % vs. 4.4 %; p = 0.0002). An exploratory analysis revealed improving responses with higher cut-offs. When the cut-off was set at ≥ 20, the difference between the two groups was even larger at 36.8 % vs. 5.0 % (p < 0.0001). Median duration of response had not been reached in patients with bTMB scores ≥ 16. Similarly, patients with high bTMB fared better than those with low bTMB concerning PFS, although not significantly so (4.6 vs. 3.7 months; HR, 0.66; p = 0.12). At 9 months, PFS rates were 37.4 % vs. 9.7 %. For OS, the data were not mature yet. When analysed by various bTMB cut-offs (≥ 10, ≥ 16, ≥ 20), both PFS and OS improved with increasing scores (Figure 2). For both endpoints, the ≥ 16 prespecified score appeared to be an inflection point that clearly separated out efficacy. Atezolizumab was well tolerated. bTMB is currently being validated in a prospective, randomised phase III trial.
Figure 2: B-F1RST study: progression-free survival forest plot according to bTMB scores
Long-term outcomes with pembrolizumab: KEYNOTE-010
The randomised, open-label, phase II/III KEYNOTE-010 study has shown superior OS activity of pembrolizumab monotherapy at two doses compared to docetaxel in patients with previously treated, PD-L1–expressing advanced NSCLC [11]. At the ESMO 2018 Congress, Herbst et al. presented updated OS and safety results with 30 additional months of follow-up as well as outcomes for patients who completed 35 cycles or 2 years of pembrolizumab treatment [12].
According to this analysis, pembrolizumab continued to prolong OS compared to docetaxel. In the population with a PD-L1 tumour proportion score (TPS) ≥ 50 %, 35 % vs. 13 % of patients were alive at 36 months (median OS, 16.9 vs. 8.2 months; HR, 0.53; p < 0.00001). For those with TPS ≥ 1 %, the respective proportions were 23 % and 11 % (median OS, 11.8 vs. 8.4 months; HR, 0.69; p < 0.00001).
Seventy-nine patients completed 35 cycles or 2 years of treatment. In this group, 95 % had complete or partial responses according to independent central review. Responses were ongoing in 64 % at the time of the analysis. Median duration of response had not been reached yet; this also applied to median PFS and median OS. At 36 months, 98.7 % of patients were alive, and 70.3 % were both alive and progression-free. Twenty-five patients experienced disease progression after stopping 35 cycles or 2 years of treatment. Of these, 14 were able to start a second course of pembrolizumab therapy, with partial responses resulting in 43 %. Stable disease occurred in 36 %. The long-term safety profile of pembrolizumab treatment including in patients who completed 35 cycles or 2 years of treatment proved manageable.
REFERENCES
- Howington JA et al., Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(5 Suppl): e278S-e313S
- Pignon JP et al., Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008; 26(21): 3552-3559
- NSCLC Meta-analysis Collaborative Group, Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014; 383: 1561-1571
- Forde PM et al., Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018; 378(21): 1976-1986
- Cascone T et al., NEOSTAR: neoadjuvant nivolumab or nivolumab plus ipilimumab for resectable non-small cell lung cancer. ESMO 2018, abstract LBA49
- Antonia SJ et al., Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017; 377(20): 1919-1929
- Antonia SJ et al., Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018 Sep 25. doi: 10.1056/NEJMoa1809697
- Faivre-Finn C et al., Exploratory analyses of overall survival in PACIFIC. ESMO 2018, abstract 13630
- Cappuzzo F et al., IMpower130: efficacy and safety from a randomised phase 3 study of carboplatin and nab-paclitaxel with or without atezolizumab in 1L advanced non-squamous NSCLC. ESMO 2018, abstract LBA53
- Kim ES et al., Primary efficacy results from B-F1RST, a prospective phase II trial evaluating blood-based tumour mutational burden (bTMB) as a predictive biomarker for atezolizumab in 1L non-small cell lung cancer (NSCLC). ESMO 2018, abstract LBA55
- Herbst RS et al., Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387(10027): 1540-1550
- Herbst RS et al., Long-term survival in patients with advanced NSCLC in the KEYNOTE-010 study overall and in patients who completed 2 years of pembrolizumab. ESMO 2018, abstract LBA63
More posts
Interview: Several reasons support sequencing of EGFR TKI treatment
The first-line EGFR TKI choice in patients with EGFR-mutated NSCLC has been under debate ever since the results of the FLAURA were reported. From the current point of view, what are the limitations of this study?
EGFR-mutant lung cancer: what’s new with respect to activity and resistance?
In patients with stage IIIA-N2 NSCLC, current multimodal treatment options include definitive chemoradiotherapy, surgery followed by adjuvant chemotherapy, or neoadjuvant treatment followed by surgical resection. The standard first-line EGFR tyrosine kinase inhibitor (TKI) erlotinib has already demonstrated feasibility in the neoadjuvant treatment setting of stage IIIA-N2 NSCLC.
Checkpoint inhibition excels in all treatment lines
Patients with early and locally advanced (stage I-IIIA) non–small-cell lung cancer (NSCLC) usually undergo surgery, but long-term outcomes leave much to be desired. After surgery alone, the recurrence rate is substantial at more than 50 %. Perioperative chemotherapy as a means to prevent disease recurrence only confers a 5 % improvement in 5-year survival compared to sole surgery.
Preface – ESMO 2018
The ESMO Congress represents the leading international oncology event in Europe. This year’s conference that took place from 19th to 23rd October in Munich, Germany, was held under the tagline “Securing access to optimal cancer care”. Approximately 25,000 participants including experts from various oncology disciplines, healthcare policy makers, and patient advocates convened from all over the world to discuss innovations and the major challenge of turning new insights into actual improvements in cancer patient care.