Expansion of treatments and insights in the early-stage disease setting
The management of patients with stage I-III non–small-cell lung cancer (NSCLC) is still characterized by a high unmet medical need as up to 60 % experience disease relapse despite treatment with curative intent [1]. IMpower010 was the first phase III study of cancer immunotherapy to demonstrate a disease-free survival (DFS) benefit in the adjuvant situation after complete resection and platinum-based chemotherapy. The interim DFS analysis showed that compared to best supportive care (BSC), atezolizumab 1,200 mg Q3W for 16 cycles gave rise to significant DFS benefits in the PD-L1–positive (i.e., TC ≥ 1 %) stage II-IIIA and all-randomized stage II-IIIA populations (HRs, 0.66 and 0.79, respectively) [2]. In the intent-to-treat (ITT) population (i.e., all-randomized stage IB-IIIA patients), the significance boundary for DFS was not crossed.
At ESMO 2021, Felip et al. reported sites of disease relapse and post-relapse treatment in IMpower010 at the time of the interim DFS analysis [3]. Moreover, DFS was explored by PD-L1 expression in the all-randomized stage II-IIIA population; this demonstrated that atezolizumab-treated patients with TC ≥ 50 % experienced the greatest risk reduction (HR, 0.43), while those with TC 1-49 % did not benefit significantly (HR, 0.87).
Consistent benefits in IMpower010
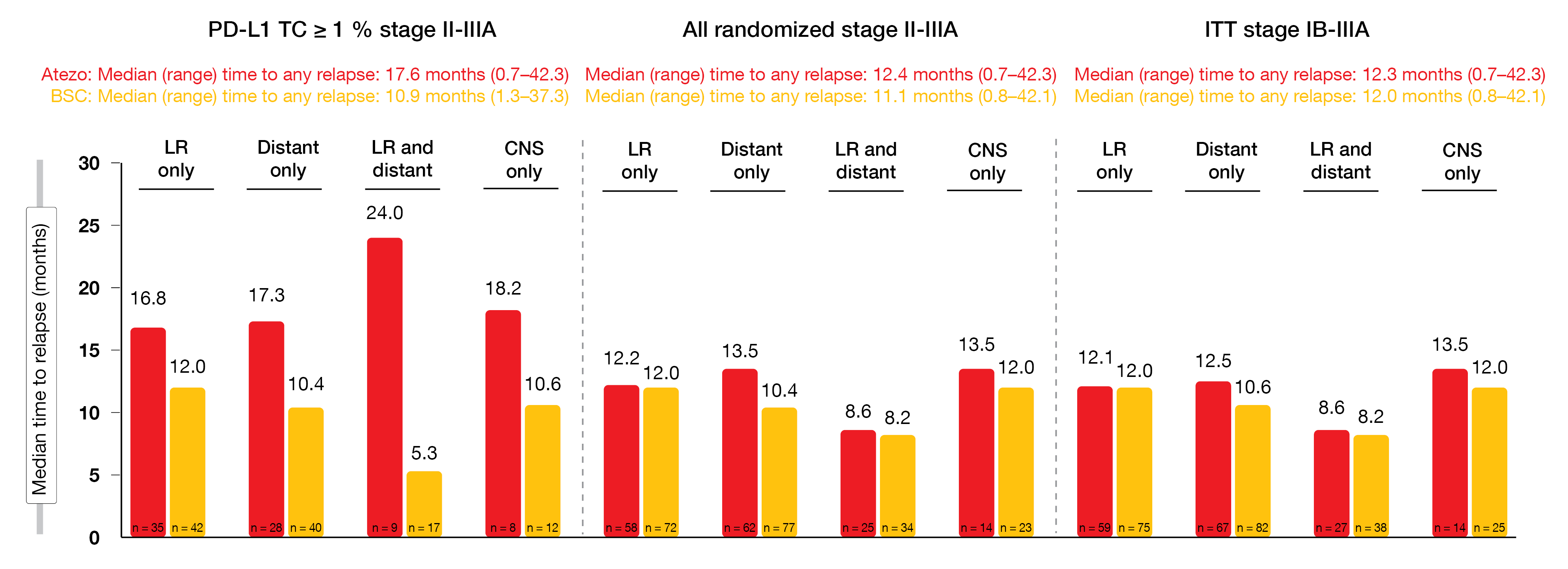
Similar patterns of relapse were observed across the study arms. In the PD-L1–positive stage II-IIIA population, locoregional relapses only occurred more commonly in both arms (47.9 % and 41.2 % for atezolizumab and BSC, respectively) than distant relapses only (38.4 % and 39.2 %, respectively). Conversely, in the all-randomized stage II-IIIA and ITT stage IB-IIIA populations, distant metastases emerged slightly more often than locoregional relapses irrespective of treatment. CNS relapses only were seen in 9.0 % to 12.3 % across all groups. Time from randomization to relapse appeared to favor atezolizumab therapy in the PD-L1–positive stage II-IIIA population for all relapse categories, while the differences were small in the all-randomized stage II-IIIA and ITT stage IB-IIIA populations (Figure 1).
With respect to post-relapse treatment, the analysis revealed higher rates of immunotherapy in the BSC arms of the three populations compared to the experimental arms, while no differences were observed regarding other types of subsequent treatment including chemotherapy and targeted agents. Likewise, post-relapse use of surgery and radiotherapy was similar. The authors concluded that longer follow-up is warranted and might reveal differences in relapse patterns and treatment options.
Another exploratory analysis of the IMpower010 study assessed prior therapies, including the type of surgery, and their potential impact on DFS outcome [4]. Within the randomized ITT population (n = 1,005), the majority of patients had undergone lobectomy (78.1 %) and lymph node dissection (80.7 %), and most had received 4 cycles of adjuvant chemotherapy. Median time from surgery to the first administration of atezolizumab or BSC was similar (5.2 and 5.1 months, respectively). The forest plots revealed that adjuvant atezolizumab, as compared to BSC, improved DFS in the PD-L1 TC ≥ 1 % stage II-IIIA and all-randomized stage II-IIIA populations across most disease stages, in patients with nodal involvement, and across most surgery types and chemotherapy regimens.
Figure 1: Time from randomization to relapse for the PD-L1 TC ≥ 1 % stage II-IIIA, all randomized stage II-IIIA, and ITT stage IB-IIIA populations included in IMpower010. LR, locoregional; CNS, central nervous system
NADIM: neoadjuvant use of nivolumab
The single-arm, phase II NADIM trial assessed nivolumab 360 mg Q3W in addition to 3 cycles of neoadjuvant administration of paclitaxel and carboplatin in patients with resectable stage IIIA N2 or T4N0/N1 tumors. Surgery was conducted in the third or fourth week from day 21 of cycle 3. The adjuvant treatment comprised nivolumab for 1 year. In terms of the primary endpoint, which was progression-free survival (PFS) at 24 months, the primary analysis yielded a 77.1 % rate, thus supporting the addition of neoadjuvant nivolumab to platinum-based chemotherapy [5].
At WCLC 2021, the 3-year overall survival (OS) analysis was reported for the ITT (n = 46) and per-protocol populations (PP; n = 37) [6]. The latter included the patient group that received adjuvant therapy. NADIM showed promising survival results, with 36-month OS rates of 81.9 % and 91.0 % in the ITT and PP populations, respectively. This markedly exceeded the historical 3-year OS rates that have remained at approximately 30 % over the past decades. At 42 months, 78.9 % and 87.3 % of patients, respectively, were alive. The 42-month PFS rates amounted to 69.6 % and 81.1 %, respectively.
An exploratory analysis was conducted to elucidate the predictive potential of response parameters. While clinical responses based on CT scans did not predict survival outcomes, pathological complete response and circulating tumor DNA clearance (i.e., lack of detectable ctDNA at the end of neoadjuvant treatment) significantly predicted long-term survival.
The addition of nivolumab to neoadjuvant chemotherapy did not adversely affect the safety profile. For the neoadjuvant and adjuvant treatment periods alike, most treatment-related adverse events (TRAEs) were grade 1 or 2, and no fatal TRAEs occurred.
PACIFIC under real-world conditions
The phase III PACIFIC trial has established consolidation treatment with durvalumab as the standard of care in patients with unresectable stage III NSCLC who did not develop disease progression after concurrent chemoradiotherapy (CRT). Robust and long-lasting OS and PFS benefits were achieved in this study, with one third of patients remaining progression-free after 5 years of follow-up [7]. At present, the international, observational PACIFIC-R study is investigating the real-world effectiveness of the PACIFIC regimen. The analysis presented at ESMO 2021 included a total of 1,399 patients with unresectable stage III NSCLC who had been recruited into an early access program regardless of their tumor PD-L1 expression status at 290 active sites in 11 countries [8].
After a median treatment duration of approximately 11 months, median real-world PFS with durvalumab was higher than the PFS reported in the PACIFIC trial (21.7 vs. 16.9 months). The authors pointed out that inaccuracies relating to the collection of real-world data limit this comparison; for instance, RECIST criteria are being used in a heterogeneous fashion across countries, and assessments for progression might have been impaired by the COVID-19 pandemic. Nevertheless, the efficacy of durvalumab after CRT in the analyzed subgroups was generally consistent with previous data from the PACIFIC trial [9]. Real-world PFS observed with durvalumab consolidation therapy was longer in stage IIIA disease than in stage IIIB/C disease (23.7 vs. 19.2 months), in the PD-L1–positive group compared to the PD-L1–negative group (22.4 % vs. 16.3 months), after concurrent CRT compared to sequential CRT (23.7 vs. 19.4 months), and in patients with non-squamous tumors vs. those with squamous tumors (25.3 vs. 14.7 months).
Pneumonitis/interstitial lung disease was the most common AE leading to temporary treatment interruption (5.2 %) and permanent discontinuation (9.5 %). Eighteen percent of patients developed pneumonitis that was mostly mild or moderate. Corticosteroid administration was required in 71.3 % of events. The rates of treatment discontinuation due to AEs and disease progression (16.7 % and 26.9 %, respectively) were consistent with those from PACIFIC (15.4 % and 31.3 %, respectively [7]). Overall, durvalumab consolidation therapy after CRT for approximately 11 months proved effective in a large, real-world cohort.
Combinations of durvalumab with novel agents
Combined immunomodulatory consolidation with durvalumab plus other agents in the PACIFIC setting is being explored with the aim of further improving patient outcomes. The global, open-label, randomized, phase II COAST study is testing durvalumab alone or together with either the anti-CD73 antibody oleclumab or the anti-NKG2A antibody monalizumab. Radiotherapy induces expression of CD73 and the NKG2A ligand HLA-E that inhibit antitumor immune response [10-12]. Combinations of radiotherapy and CD37/NKG2A-targeted agents with or without checkpoint inhibitors have shown increased antitumor activity in preclinical models [10, 11].
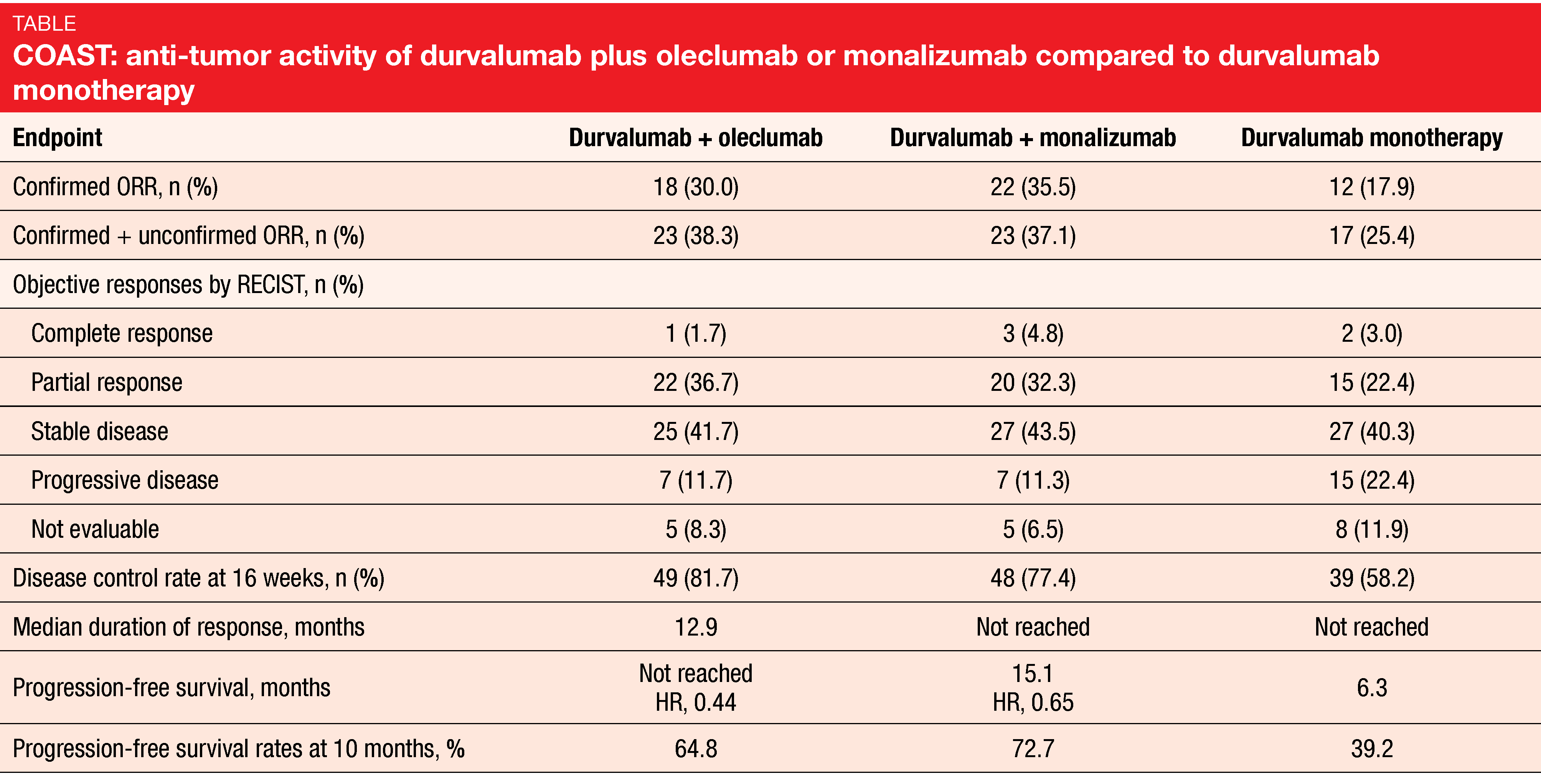
The three-arm COAST study that is being conducted at 82 sites in 9 countries is comparing durvalumab 1,500 mg plus oleclumab 3,000 mg Q4W (n = 60) with durvalumab 1,500 mg Q4W plus monalizumab 750 mg Q2W (n = 62) and durvalumab 1,500 mg Q4W monotherapy (n = 67). The objective response rate (ORR) constituted the primary endpoint. According to the interim data presented at ESMO 2021, both oleclumab and monalizumab can provide additional clinical benefit when combined with durvalumab [13]. Both combinations numerically increased ORR and gave rise to significant PFS improvement vs. durvalumab alone (Table). PFS benefits with both combinations were observed across various subgroups.
The safety profiles were consistent across arms, with similar rates of AEs of special interest including pneumonitis. No new safety signals emerged in either combination arm. Overall, COAST is the first randomized phase II study to show evidence of improved outcomes with novel immunotherapy combinations in the PACIFIC setting. These data support further evaluation of the combined regimens in a registration-intent trial.
GEMSTONE-301: sugemalimab
Another study investigating consolidation treatment in the PACIFIC setting is the randomized, double-blind, placebo-controlled, phase III GEMSTONE-301 trial. The anti–PD-L1 antibody sugemalimab is being tested at a dose of 1,200 mg Q3W (n = 255) against placebo (n = 126) for up to 24 months in Chinese patients with unresectable stage III NSCLC who have not developed progression after concurrent or sequential CRT. GEMSTONE-301 is the first phase III study to evaluate an anti-PD-(L)1 agent after both concurrent and sequential treatment in this setting based on the observation that concurrent CRT is not accessible everywhere and can confer significant toxicity. Patient comorbidities and lack of access often limit its use in the real-world setting. Two thirds and one third of the population included in GEMSTONE-301 had received concurrent and sequential CRT, respectively.
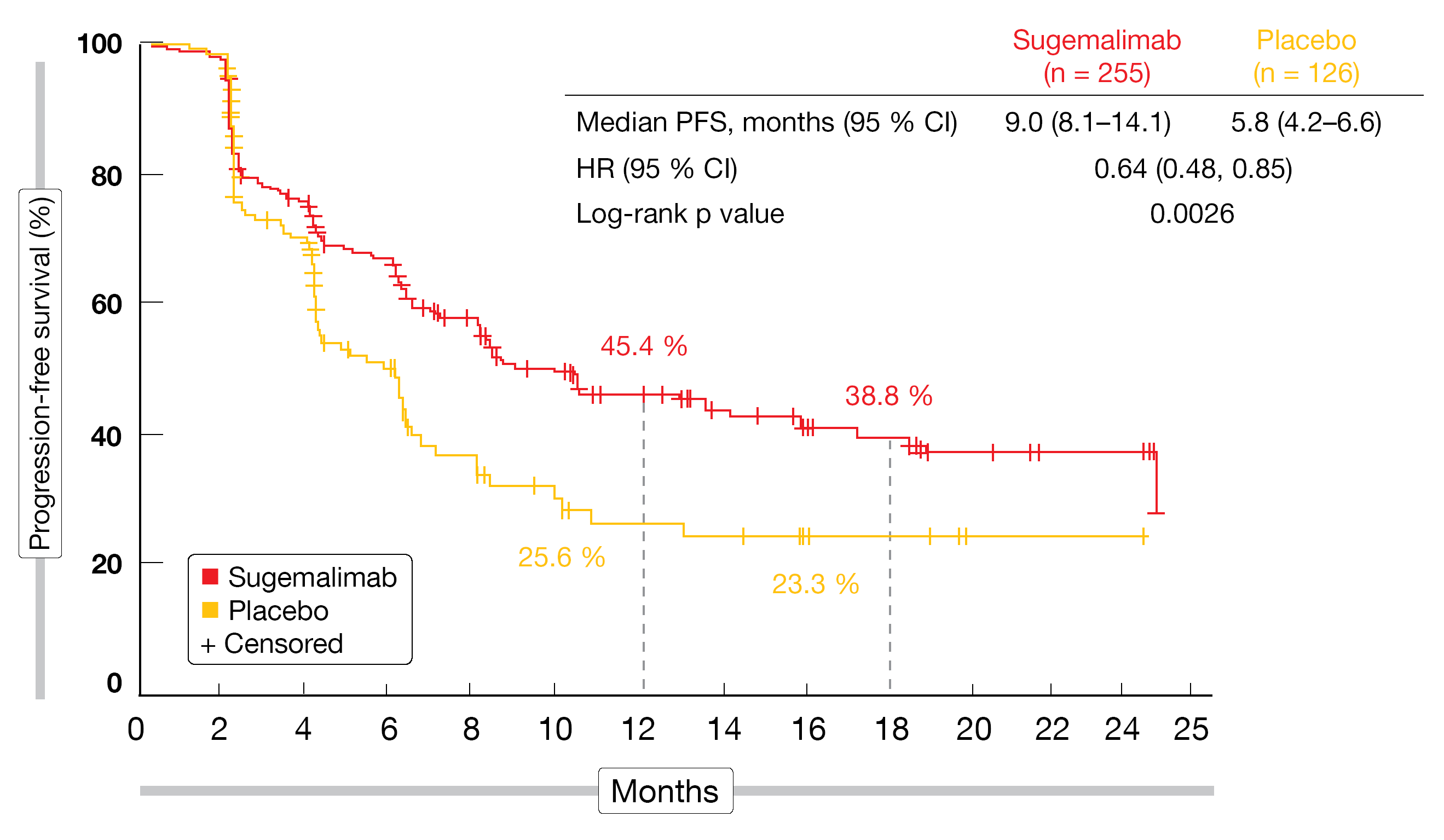
PFS by blinded independent review was defined as the primary endpoint. According to the pre-planned interim analysis reported by Wu et al. at ESMO 2021, sugemalimab gave rise to a statistically significant and clinically meaningful PFS improvement (9.0 vs. 5.8 months; HR, 0.64; p = 0.0026; Figure 2) [14]. In the group after concurrent CRT, median PFS was 10.5 vs. 6.4 months (HR, 0.66), and in the patients after sequential CRT, this was 8.1 vs. 4.1 months (HR, 0.59). The OS data were immature at the time of the analysis, although a trend was obvious for a survival benefit with sugemalimab vs. placebo (not reached vs. 24.1 months in the overall population; HR, 0.44). A markedly greater proportion of patients treated in the experimental arm was alive at 24 months (78.0 % vs. 50.7 %).
Sugemalimab showed a favorable safety profile that was in keeping with the profile previously reported for sugemalimab monotherapy in NSCLC. Immune-related AEs occurred in 42.7 % in the experimental arm, with 4.7 % classified as grade 3-5 events. Treatment cycle delay and permanent drug discontinuation were due to treatment-emergent AEs in 32.2 % and 11.4 %, respectively. The authors noted in their summary that the findings obtained in the GEMSTONE-301 study suggest effectiveness of sugemalimab as consolidation therapy in unresectable stage III NSCLC after concurrent or sequential CRT.
Figure 2: Progression-free survival advantage with sugemalimab vs. placebo after concurrent or sequential chemoradiotherapy for unresectable stage III lung cancer
Ancillary evaluations of the LungART data
The randomized phase III LungART trial assessed the effect of conformal post-operative radiotherapy (PORT) at a dose of 54 Gy delivered over 5.5 weeks in 252 patients with completely resected NSCLC and N2 nodal involvement after pre- and/or post-operative chemotherapy. DFS constituted the primary endpoint. According to the primary analysis presented in 2020, PORT, as compared to no PORT (n = 249), only slightly increased DFS (30.5 vs. 22.8 months; HR, 0.86; p = 0.18), with 3-year DFS rates of 47.1 % vs. 43.8 % [15]. At 3 years, 66.5 % vs. 68.5 % of patients were alive. While PORT reduced the risk of mediastinal relapse, this did not apply to distant metastatic events and the likelihood of brain metastases. In their recent analysis, Le Pechoux et al. focused on the patterns of failure observed in the study and the prognostic factors for PORT efficacy [16].
This showed that mediastinal relapse occurred mainly within initially involved lymph nodes. The 3 most frequently affected sites of mediastinal relapse were the stations 4R, 2R and 7 for right-sided tumors, and the stations 7, 4L and 4R for left-sided tumors. In terms of prognostic factors for DFS, female gender, squamous histology and absence of lymph node involvement were identified as protective. Besides age and WHO performance status, the burden of nodal disease as well as the quality of resection were relevant for DFS. Mediastinal-relapse–free survival at 3 years was significantly longer in the PORT arm irrespective of the burden of nodal disease and the presence of extracapsular extension. The authors concluded that personalized use of PORT should be based on prognostic factors of relapse and a joint assessment of toxicity and efficacy of this treatment approach.
REFERENCES
- Vansteenkiste J et al., Current status of immune checkpoint inhibition in early-stage NSCLC. Ann Oncol 2019; 30(8): 1244-1253
- Wakelee H et al., IMpower010: Primary results of a phase III global study of atezolizumab versus best supportive care after adjuvant chemotherapy in resected stage IB-IIIA non-small cell lung cancer (NSCLC). J Clin Oncol 2021; 39(suppl 15): 8500
- Felip E et al., IMPower010: sites of relapse and subsequent therapy from a phase 3 study of atezolizumab vs best supportive care after adjuvant chemotherapy in resected stage IB-IIIA NSCLC. ESMO 2021, LBA9
- Altorki N et al., IMpower010: characterization of stage IB-IIIA NSCLC patients by type and extent of therapy prior to adjuvant atezolizumab. WCLC 2021, PL02.05
- Provencio M et al., Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020; 21(11): 1413-1422
- Provencio M et al., Long term survival in operable stage IIIA NSCLC patients treated with neoadjuvant nivolumab plus chemotherapy – NADIM study. WCLC 2021, OA20.01
- Spigel DR et al., Five-year survival outcomes with durvalumab after chemoradiotherapy in unresectable stage III NSCLC: An update from the PACIFIC trial. J Clin Oncol 39, 2021 (suppl 15; abstr 8511)
- Girard N et al., PACIFIC-R real-world study: treatment duration and interim analysis of progression-free survival in unresectable stage III NSCLC patients treated with durvalumab after chemoradiotherapy. ESMO 2021, 1171M0
- Antonia SJ et al., Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017; 377(20): 1919-1929
- Wennerberg E et al., CD73 blockade promotes dendritic cell infiltration of irradiated tumors and tumor rejection. Cancer Immunol Res 2020; 8(4): 465-478
- Tsukui H et al., CD73 blockade enhances the local and abscopal effects of radiotherapy in a murine rectal cancer model. BMC Cancer 2020; 20(1): 411
- Nguyen AM et al., Upregulation of CD73 confers acquired radioresistance and is required for maintaining irradiation-selected pancreatic cancer cells in a mesenchymal state. Mol Cell Proteomics 2020; 19(2): 375-389
- Martinez-Marti A et al., COAST: an open-label, phase 2, multidrug platform study of durvalumab alone or in combination with novel agents in patients with locally advanced, unresectable, stage III NSCLC. ESMO 2021, LBA42
- W Y-L et al., GEMSTONE-301: a randomized, double-blind, placebo-controlled, phase 3 study of sugemalimab in patients with unresectable stage III non-small cell lung cancer without progression after concurrent or sequential chemoradiotherapy. ESMO 2021, LBA43
- Le Pechoux C et al., An international randomized trial comparing post-operative radiotherapy (PORT) to no PORT, in patients with completely resected NSCLC and mediastinal N2 involvement. ESMO 2020, LBA3_PR
- Le Pechoux C et al., An international randomized trial comparing post-operative conformal radiotherapy (PORT) to no PORT in patients with completely resected non-small cell lung cancer and mediastinal N2 involvement. ESMO 2021, 11700
© 2021 Springer-Verlag GmbH, Impressum