Innovative and established agents across a range of targets
Anti-HER2 treatment
DESTINY-Lung01: robust effects of T-DXd
HER2 mutations constitute the predominant driver aberration in approximately 3 % of non-squamous NSCLC cases [1, 2]. While approved HER2-targeted therapies for patients with NSCLC are still lacking, the anti-HER2 antibody-drug conjugate trastuzumab deruxtecan (T-DXd) has been licensed in various countries for use in other HER2-positive entities.The international, 2-cohort, phase II DESTINY-Lung01 study investigated T-DXd in patients with HER2-overexpressing and HER2-mutated, metastatic NSCLC who had relapsed on or were refractory to standard treatment. At ESMO 2021, Li et al. presented the primary analysis of the fully enrolled HER2-mutant group (i.e., Cohort 2) that contained a total of 91 patients treated with T-DXd 6.4 mg/kg Q3W [3]. Approximately 95 % of them had received platinum-based chemotherapy, 65.9 % anti-PD-(L)1 therapy, and 62.6 % both.
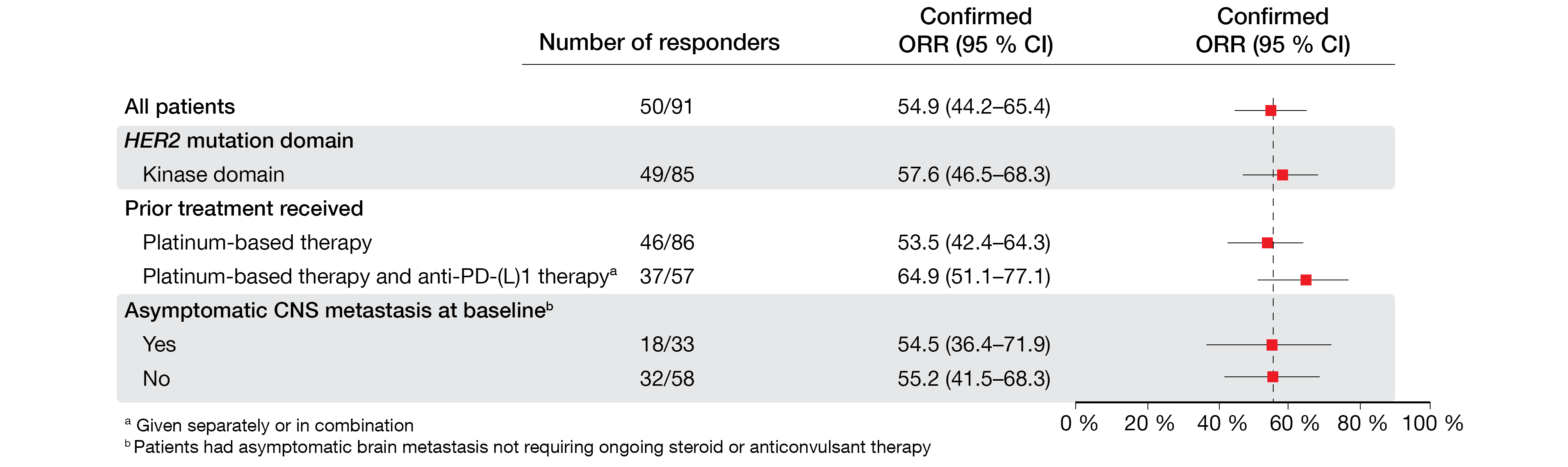
T-DXd showed robust and durable anticancer activity in these patients. The objective response rate (ORR) was 54.9 %, and 92.3 % achieved clinical benefit. Responses were consistently observed across subgroups, including patients with stable CNS lesions (Figure 1), and lasted for a median of 9.3 months. Exploratory analyses demonstrated anticancer activity across different HER2 mutation subtypes, as well as in patients with no detectable HER2 expression or HER2 gene amplification. Median progression-free survival (PFS) and overall survival (OS) amounted to 8.2 and 17.8 months, respectively.
The most common treatment-emergent AEs (TEAEs) associated with discontinuation were investigator-reported pneumonitis (13.2 %) and interstitial lung disease (ILD; 5.5 %). Seventy-five percent of adjudicated drug-related ILD/pneumonitis cases were grade 1 or 2. Nevertheless, the authors pointed out that these events remain an important identified risk that calls for effective early detection and management. TEAE-related dose reductions occurred in 34.1 % and were mainly due to nausea (11.0 %) and fatigue (8.8 %).
In their conclusion, the authors noted that DESTINY-Lung01 provides compelling evidence of positive benefit-risk balance with T-DXd in the second- and later-line settings. The data support the implementation of T-DXd as a potential new treatment standard. Meanwhile, the 5.4 mg/kg dose is being explored in the DESTINY-Lung02 trial to establish the optimal dosing regimen in patients with HER2-mutant NSCLC.
Figure 1: Consistent responses across subgroups observed with trastuzumab deruxtecan in patients with HER2-mutated NSCLC
Poziotinib in HER2 exon 20 insertions
The multi-cohort global ZENITH20 trial is assessing the pan-HER tyrosine kinase inhibitor (TKI) poziotinib in various settings including EGFR or HER2 exon 20 insertion mutations, atypical EGFR or HER2 mutations, and after failure of osimertinib. In Cohort 4 of the study, first-line poziotinib is being investigated in patients with HER2 exon 20 insertions; 48 of these are receiving 16 mg once daily, while enrollment is still ongoing in the group of 23 patients treated with 8 mg twice daily. Cornelissen et al. reported preliminary efficacy and safety data for the once daily dosing group within Cohort 4 at ESMO 2021 [4].
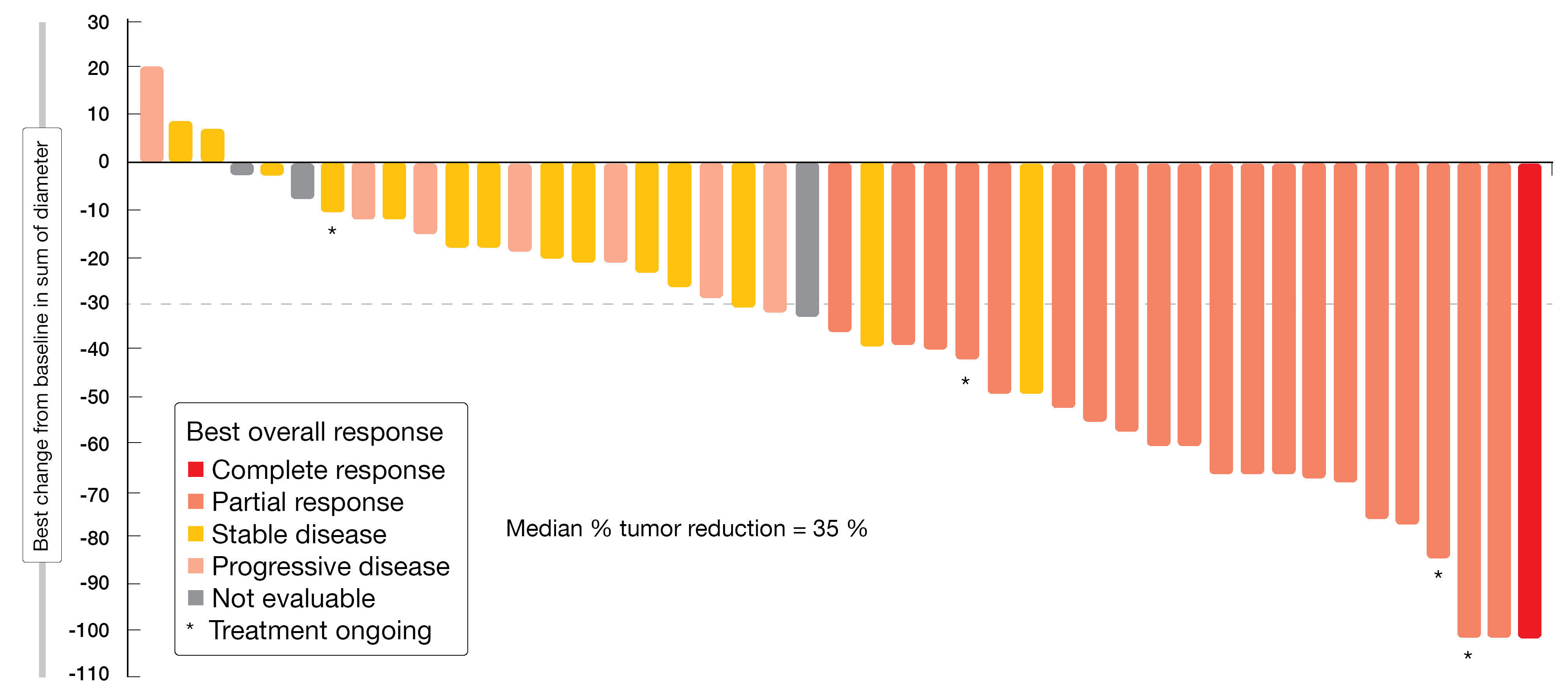
With respect to objective response, which had been defined as the primary endpoint, the treatment induced a centrally reviewed confirmed rate of 43.8 %. Disease control occurred in 75.0 %. Eighty-eight percent of patients experienced tumor reduction (Figure 2). Median PFS was 5.6 months, with 26 % of patients being alive and progression-free beyond 12 months. Responses lasted for a median of 5.4 months; here, the upper range was > 19.1 months. Poziotinib showed a manageable toxicity profile that was in line with previous poziotinib studies and other second-generation EGFR TKIs. Diarrhea, rash and stomatitis were observed as the most common AEs. AEs leading to dose reductions and permanent discontinuation occurred in 77 % and 13 %, respectively. Overall, once-daily poziotinib treatment demonstrated clinically meaningful efficacy in treatment-naïve patients with NSCLC harboring HER2 exon 20 insertion mutations.
Figure 2: Reductions in target tumor size from baseline in patients with HER2 exon 20 insertion receiving poziotinib
EGFR/MET inhibition: amivantamab
Companion diagnostics & combination with lazertinib
Based on the ongoing phase I CHRYSALIS trial, the EGFR/MET bispecific antibody amivantamab has received US approval for the treatment of patients with EGFR exon 20 insertion-positive NSCLC who have progressed on or after platinum-based chemotherapy. CHRYSALIS is assessing amivantamab in various settings as monotherapy and combined with the third-generation EGFR TKI lazertinib. A bridging study was conducted to clinically validate two potential NGS-based companion diagnostics, the plasma-based Guardant360® CDx and the tissue-based Oncomine™ Dx Target Test, for the detection of EGFR exon 20 insertion variants. According to the results presented at WCLC 2021, these tests showed a high degree of concordance, and both can be used to accurately identify patients for amivantamab therapy [5].
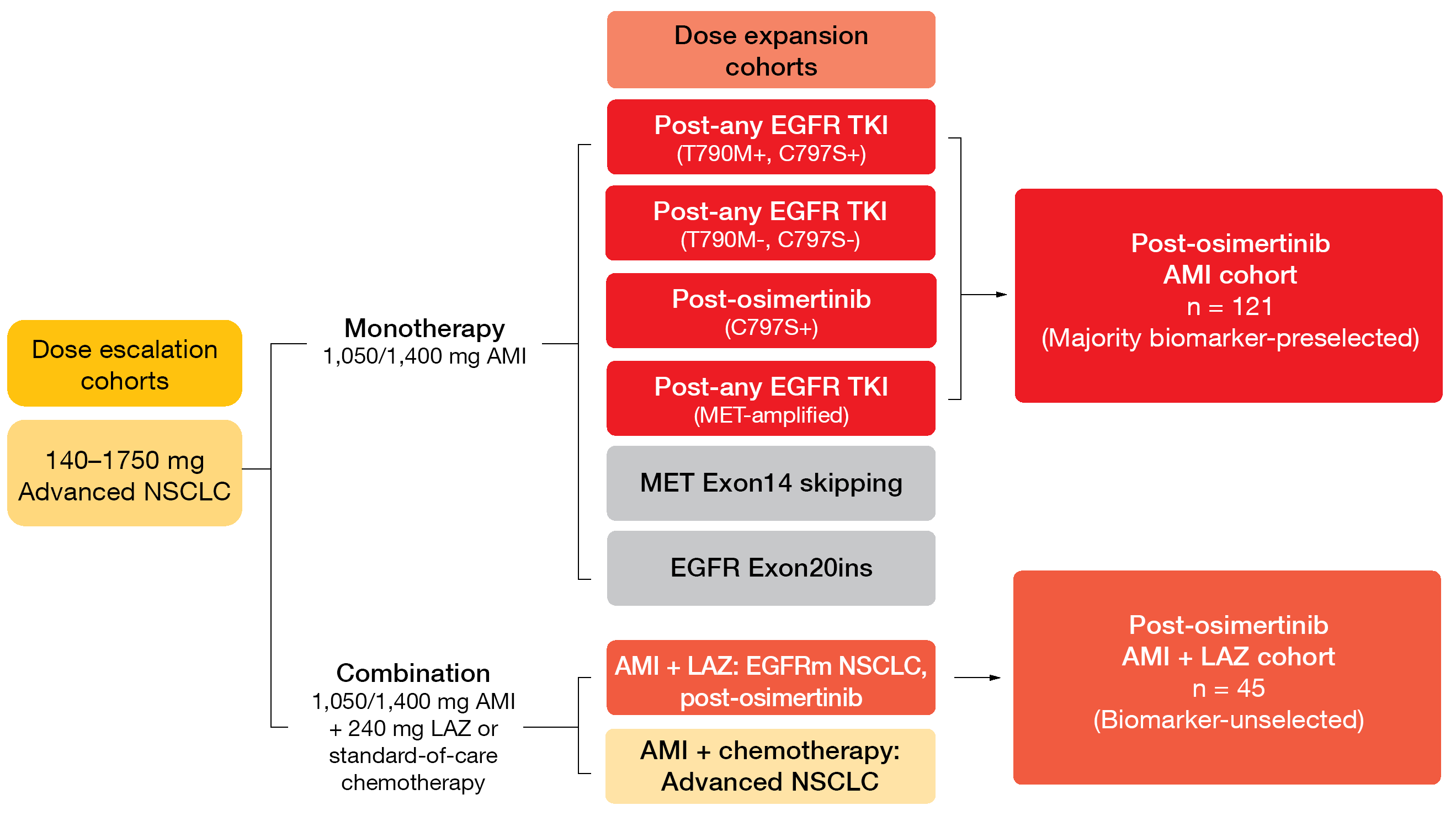
At ESMO 2021, Leighl et al. reported CHRYSALIS data on the contribution of the addition of lazertinib to amivantamab after osimertinib failure [6]. The amivantamab plus lazertinib and amivantamab monotherapy cohorts included 45 and 121 patients, respectively (Figure 3). Indeed, the combination appeared to have higher activity than the monotherapy. The addition of lazertinib resulted in a numerically higher ORR compared to amivantamab alone (36 % and 19 %, respectively) and longer duration of response (9.6 and 5.9 months, respectively). Responses for ≥ 6 months occurred in 69 % and 39 %, respectively. CNS protection was potentially improved in the combined cohort considering the even lower CNS progression rate of 7 % compared to 17 % in the amivantamab monotherapy cohort.
The safety profile for both monotherapy and combination therapy was consistent with previously reported experience. Amivantamab plus lazertinib is being evaluated in multiple EGFR NSCLC populations in the CHRYSALIS-2 study and the phase III MARIPOSA trial.
Figure 3: Design of the CHRYSALIS trial investigating amivantamab (AMI) alone and in combination with lazertinib (LAZ)
Findings in MET exon 14 skipping mutations
Spira et al. reported the first results for amivantamab obtained in the cohort of patients enrolled in the CHRYSALIS study whose tumors harbored MET exon 14 skipping (METex14) mutations [7]. These mutations occur in approximately 3 % of NSCLC cases [8] and are amenable to treatment with MET TKIs, although emergence of resistance is a major concern here. The METex14 population included in CHRYSALIS is still being recruited; 19 patients with metastatic or unresectable NSCLC who had progressed after standard-of-care treatment or declined it were included in the analysis.
Among 14 response-evaluable patients, 9 (64 %) developed partial responses on amivantamab therapy, with 5 confirmed and 4 pending confirmation. Activity was observed in both treatment-naïve and previously treated patients; this included 7 individuals previously treated with MET TKIs two of whom showed potential resistance mechanisms. Median duration of response had not been reached, and 11 of the 14 response-evaluable patients remained on treatment.
The safety profile of amivantamab in this patient subgroup was consistent with the previously reported profile observed in patients with EGFR-mutated NSCLC. According to the authors, this first report of amivantamab in METex14-positive NSCLC confirms the bispecific targeting action of this agent, with monotherapy activity demonstrated in both EGFR-driven and MET-driven NSCLC.
Anti-c-MET ADC telisotuzumab vedotin
An ongoing single-arm, 2-stage, adaptive phase II study is evaluating the first-in-class anti-c-MET antibody drug conjugate telisotuzumab vedotin in the setting of previously treated, locally advanced or metastatic NSCLC with c-Met protein overexpression. The trial contains three cohorts based on histology and EGFR mutation status: a squamous cohort, a non-squamous cohort with EGFR mutations, and a non-squamous cohort with EGFR wildtype. Each of the non-squamous groups was further divided into two subgroups that show either high or intermediate c-Met expression. After this first stage, a decision is made based on ORR as to which patients to continue into the expansion stage. Telisotuzumab vedotin is administered at a dose of 1.9 mg/kg Q2W.
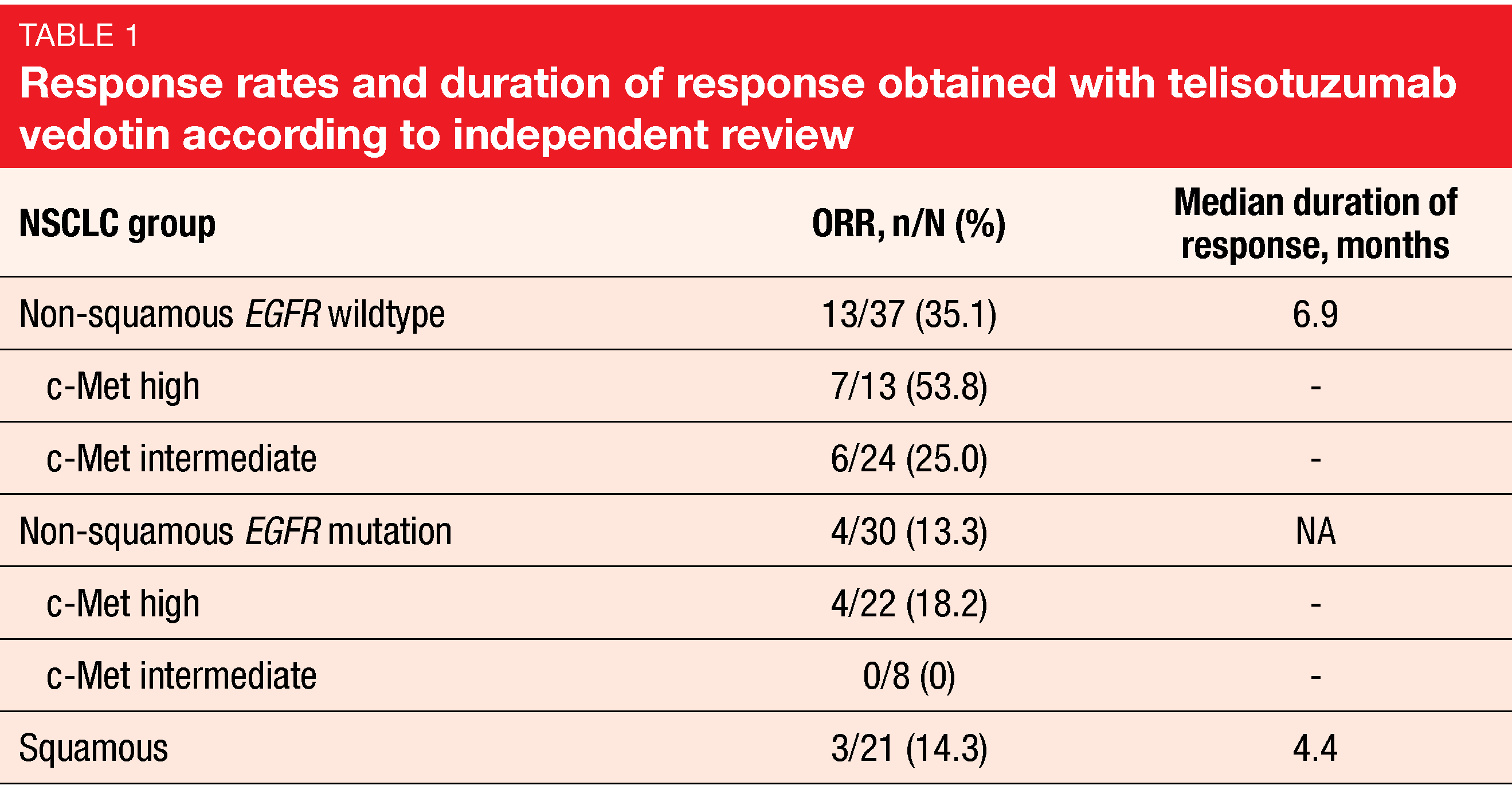
The interim analysis reported by Camidge et al. at ESMO 2021 related to 37 and 31 patients in the non-squamous EGFR wildtype and EGFR mutation populations, respectively, and 22 patients in the squamous cohort [9]. C-Met expression based on H score was lower in the EGFR wildtype population (median, 225) than in the group with EGFR mutations (265), while the lowest score was observed in the squamous cohort (164). Among the non-squamous patients, the majority of those with EGFR mutation showed high c-Met expression (71 %), whereas intermediate c-Met expression prevailed in the group with EGFR wildtype (65 %).
Telisotuzumab vedotin demonstrated a promising ORR of 35.1 % in the non-squamous EGFR wildtype cohort (Table 1). Patients in this cohort with high c-Met expression achieved responses in 53.8 %, thus obtaining the highest ORR of all groups, although the ORR was also clinically significant in those with intermediate c-Met expression. Based on pre-specified criteria, this cohort has expanded into stage 2 enrollment. All other groups responded to a modest extent. The most common serious TEAEs were pneumonia (5 %), malignant neoplasm progression (4 %), and pneumonitis (4 %). Grade ≥ 3 TEAEs occurred in 44 % of patients. Enrollment has been discontinued in the squamous cohort but will continue in the EGFR-mutated cohort until the next interim analysis.
EGFR exon 20 insertions
Mobocertinib after successful TKI treatment
The EGFR TKI mobocertinib that specifically targets EGFR exon 20 insertions has been granted Breakthrough Therapy designation for the treatment of NSCLC patients with EGFR exon 20 insertion mutations after progression on platinum-based chemotherapy (in the United States) or after prior chemotherapy (in China) based on preliminary phase I/II results [10]. At ESMO 2021, Spira et al. presented efficacy and safety data from an expansion cohort of patients who had progressed after an objective response or stable disease for ≥ 6 months on any prior EGFR TKI therapy in the phase I/II study [11]. Twenty patients received mobocertinib 160 mg once daily. Prior TKI therapies included poziotinib, osimertinib, afatinib, and erlotinib. In 55 %, EGFR TKIs had been administered as the most recent prior treatment, including poziotinib (n = 7), osimertinib (n = 3), and an investigational TKI (n = 1). Median time on prior TKI therapy was 7.8 months.
The treatment with mobocertinib provided a clinically meaningful benefit in this patient group. After a median follow-up of 14.2 months, confirmed ORR and disease control rate were 40 % and 90%, respectively. Responses lasted for a median of 13.0 months. Median PFS was 7.3 months, and median OS had not been reached yet. At 12 months, 78.6 % of patients were alive. The analysis further demonstrated that the safety profile resembled that in other patient cohorts from the phase I/II trial and was consistent with the broader class of EGFR TKIs. Among all-grade treatment-related AEs, diarrhea occurred most commonly (90 %), although grade ≥ 3 diarrhea emerged only in one patient. AEs led to dose reductions in 4 patients (20 %), and 2 (10 %) discontinued treatment due to AEs. The authors concluded that mobocertinib represents a potentially promising treatment option in patients with EGFR exon 20 insertion-positive metastatic NSCLC including those who experienced objective responses on prior EGFR TKI therapy.
Novel inhibitor DZD9008
The selective, irreversible EGFR/HER2 inhibitor DZD9008 is being developed for the treatment of patients with NSCLC and EGFR or HER2 mutations. DZD9008 demonstrated encouraging anti-tumor activity in heavily pretreated patients with different subtypes of EGFR exon 20 insertion mutations in the two ongoing phase I studies WU-KONG1 and WU-KONG2 [12]. Both of these trials contain several dose escalation and dose expansion cohorts; in addition, WU-KONG1 has a food effect cohort. Overall, the safety set included 102 patients treated with at least one dose of DZD9008 50 mg to 400 mg once daily in either study. The efficacy set included 56 patients with EGFR exon 20 insertions who received DZD9008 50 mg to 400 mg once daily followed by at least one post-treatment RECIST assessment. Here, 18 and 38 patients were derived from the dose escalation and dose expansion cohorts, respectively.
The novel agent showed favorable pharmacokinetic properties with a half-life of approximately 50 hours and no apparent effect of high-fat food on exposure, among others. Regarding anti-tumor efficacy, the confirmed ORRs were 22.2 % and 44.7 % across all dose levels in the dose escalation and dose expansion cohorts, respectively. Efficacy was observed at doses ≥ 100 mg, in patients with and without baseline brain metastases, and across different EGFR exon 20 insertion subtypes. The longest treatment duration was more than 17 months. Median PFS had not been reached yet at the time of the analysis. DZD9008 was well tolerated, with a manageable AE profile. Across all dose levels, grade ≥ 3 drug-related AEs occurred in 33.3 %. AEs leading to dose reduction or discontinuation were observed in 15.7 % and 5.9 %, respectively. A phase II study evaluating DZD9008 in pretreated patients with NSCLC harboring EGFR exon 20 insertions is ongoing.
Combinations of EGFR TKIs with bevacizumab
BEVERLY: erlotinib
Studies have shown that the addition of VEGF inhibitors to first-generation EGFR TKIs can prolong PFS in EGFR-mutated, non-squamous NSCLC [13-15]. The multicenter, randomized, phase III BEVERLY trial tested the combination of the anti-VEGF antibody bevacizumab with erlotinib in the first-line treatment of patients with stage IIIB or IV, EGFR-positive NSCLC. A total of 160 individuals were randomized in a 1:1 fashion to either erlotinib 150 mg once daily plus bevacizumab 15 mg/kg Q3W or single-agent erlotinib.
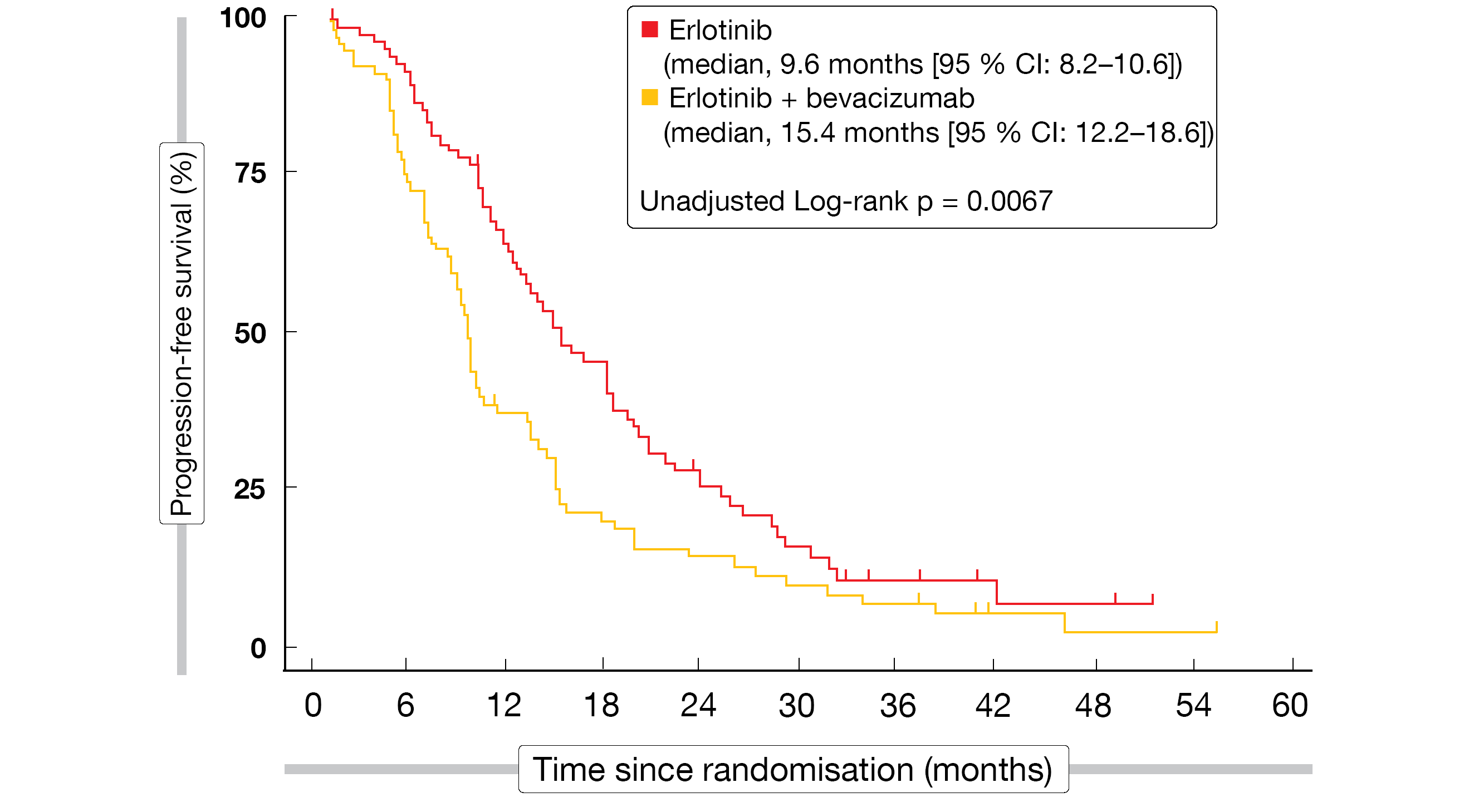
Compared to erlotinib only, the combined administration of bevacizumab plus erlotinib significantly improved PFS (15.4 vs. 9.6 months; HR, 0.66; p = 0.015; Figure 4) and ORR (70 % vs. 50 %; p = 0.01), whereas OS was prolonged in a non-significant manner (33.3 vs. 22.8 months; HR, 0.72; p = 0.132) [16]. Exploratory subgroup analyses of PFS and OS suggested that the benefit of the combination might be particularly pronounced in former and current smokers.
A quality-of-life analysis revealed no significant difference across the treatment arms in any of the items of the EORTC C30-LC13 questionnaire. With respect to time to deterioration of global health status/quality of life, patients in the experimental arm showed slightly better outcomes, although this difference was not significant. No unexpected safety signals occurred. The combination gave rise to higher rates of severe hypertension and rash. As the authors stated, bevacizumab plus erlotinib might be considered as a first-line option in patients who cannot receive osimertinib, and this regimen warrants further investigation.
Figure 4: Progression-free survival achieved with the addition of bevacizumab to erlotinib in the BEVERLY trial
WJOG9717L: osimertinib
No benefit from the addition of bevacizumab to another EGFR-targeted agent was found in the WJOG9717L study [17]. Osimertinib 80 mg once daily (n = 61) plus bevacizumab 15 mg/kg Q3W was compared with osimertinib alone (n = 61). The combination did not show superiority in terms of PFS (22.1 vs. 20.2 months; p = 0.213), ORR (82 % vs. 86 %), or OS (HR, 0.970), although the subgroup analysis indicated that ever-smokers or patients with exon 19 deletion might derive PFS benefits from the addition of bevacizumab (HRs, 0.481 and 0.622, respectively). According to the AE analysis, the combination might reduce the osimertinib-associated risk of pneumonitis, although hypertension, epistaxis and proteinuria occurred considerably more often in the experimental arm.
Real-world experience relating to afatinib use
UpSwinG: sequential treatment
In patients with EGFR-mutant NSCLC, survival outcomes substantially depend on the availability and implementation of subsequent therapy following acquired resistance to first-line therapy. As the T790M mutation is the cause of resistance to afatinib in up to 50-70 % of cases [18] and is highly sensitive to osimertinib, the sequential use of afatinib and osimertinib might help to maximize the duration of targeted treatment. In the non-interventional, global UpSwinG study, health records were analyzed to assess patient outcomes on first-line afatinib followed by second-line osimertinib after the acquisition of the T790M mutation in regular clinical practice. The analysis reported at WCLC 2021 included 191 patients who were predominantly Asian and female [19]. Approximately 14 % had brain metastases.
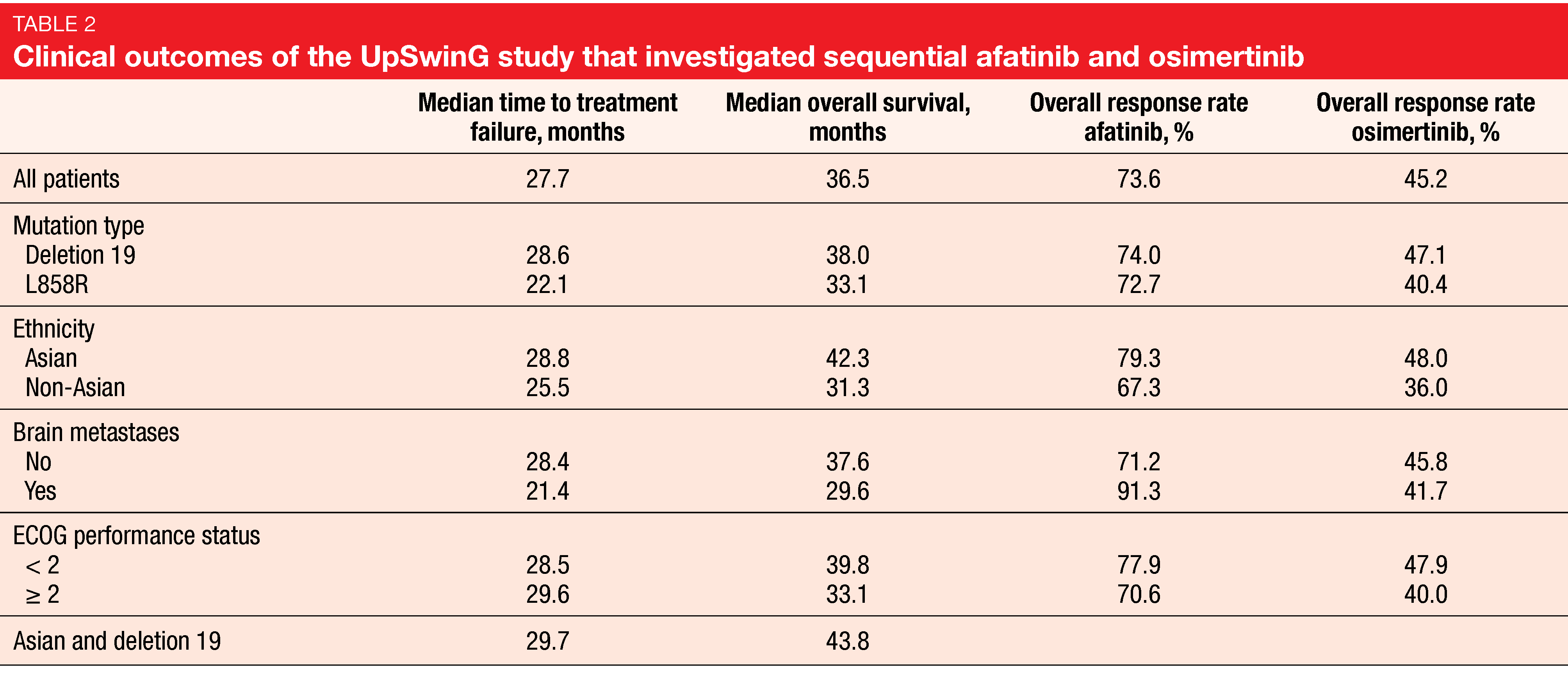
The median duration of treatment with afatinib and osimertinib was 15.1 and 9.5 months, respectively. Nearly half of the patients had at least one further treatment line after osimertinib, which mostly consisted of chemotherapy (84.5 %). Time to treatment failure, which was defined as the primary endpoint, was 27.7 months, and median OS amounted to 36.5 months (Table 2). These outcomes were consistent across subgroups including those with ECOG PS ≥ 2 and brain metastases. Median OS was longest in Asian patients (42.3 months) and Asian patients with deletion 19 mutation (43.8 months). The authors pointed out that these data are of special interest as osimertinib, as compared to first-line EGFR TKIs, has been shown not to confer significant OS benefit in Asians [20].
Overall, the results from the UpSwinG study substantiate previous studies such as GioTag that demonstrated encouraging OS of > 3 years in patients with acquired T790M who received sequential afatinib and osimertinib [21]. However, the majority of patients included in UpSwinG underwent tissue biopsy, and mutations were mainly detected based on PCR-based techniques. The authors noted that greater implementation of next-generation sequencing and liquid biopsies might increase the number of patients who are likely to benefit from targeted treatment.
Elderly patients and uncommon mutations
Brückl et al. presented the final results of a post-hoc analysis of elderly patients included in the prospective, non-interventional GIDEON study that investigated first-line afatinib treatment in routine clinical practice in Germany [22]. Forty-three percent (n = 66) of the patients treated in GIDEON were aged ≥ 70 years. Compared to those younger than 70 years, this group tended to have worse ECOG performance status and a greater number of comorbidities (Charlson Comorbidity Index ≥ 1, 36 % vs. 9 %). Nevertheless, the efficacy of treatment did not appear to be compromised. PFS outcomes were comparable in the groups aged ≥ 70 years and > 70 years, with 12-month PFS rates of 58.9 % and 43.9 %, respectively. Median OS was 30.4 and 27.4 months, respectively. Moreover, elderly patients showed a similar safety profile and incidence of grade ≥3 AEs. Dose reductions and discontinuations due to AEs were required in similar percentages.
There is a lack of clinical data on the activity of EGFR TKIs in NSCLC patients with uncommon EGFR mutations (i.e., non-deletion 19/L858R). An updated analysis of a database of patients with NSCLC and uncommon EGFR mutations treated with afatinib in randomized controlled trials and real-world practice was reported at ESMO 2021 [23]. Overall, 1,023 patients were identified; most of them were treated in clinical studies or compassionate use programs.
Afatinib demonstrated pronounced activity against major uncommon, compound, and other uncommon mutations including E709X and L747X mutations with respect to time to treatment failure and ORR. Encouraging results were observed for time to treatment failure irrespective of ethnicity (11.5 and 10.3 months in Asian and non-Asian TKI-naïve patients, respectively) and the presence of brain metastases (8.2 months). The analysis also showed activity against certain exon 20 insertions at residues A763, M766, N771, and V769, and against the osimertinib resistance mutations G724S, L718Q, L718V, and C797S. In the 15 patients who received afatinib after osimertinib, ORR and disease control rate were 36 % and 100 %, respectively.
TROP2-targeted therapy
Update of TROPION-PanTumor01
The antibody-drug conjugate datopotamab deruxtecan (Dato-DXd) has been developed to target the transmembrane glycoprotein TROP2 that is highly expressed in NSCLC regardless of genomic mutation status and has been associated with poor prognosis [24-26]. Dato-DXd was tested in the dose-escalation/dose-expansion phase I TROPION-PanTumor01 study in various tumor types. In the NSCLC cohort, 50 patients each received Dato-DXd 4 mg/kg and 6 mg/kg, while 80 were treated with 8 mg/kg.
Previous analyses of the TROPION-PanTumor01 study have demonstrated promising antitumor activity with a manageable safety profile in this heavily pretreated NSCLC cohort [27, 28]. At WCLC 2021, Garon et al. presented updated results according to which Dato-DXd continued to demonstrate highly encouraging efficacy and a manageable safety profile at all three doses [29]. The 6 mg/kg dose, which had been selected for further development, gave rise to an ORR of 28 % and median duration of response of 10.5 months. Most responses were durable over time. TEAEs were primarily non-hematologic (i.e., nausea, stomatitis, alopecia, fatigue), and were mostly classified as mild or moderate. ILD that was adjudicated as drug-related occurred in 6 % in the 6 mg/kg cohort. The phase III TROPION-Lung01 trial is currently evaluating Dato-DXd for the treatment of NSCLC.
Dato-DXd in patients with actionable aberrations
The TROPION-PanTumor01 subset analysis presented at ESMO 2021 related to patients with actionable genomic alterations (AGAs) [30]. As is known, this group derives limited benefit from existing therapies once TKIs and platinum-based chemotherapy have failed [31, 32]. The AGA subset included in the NSCLC cohort of TROPION-PanTumor01 comprised 34 patients with EGFR mutations (85 %), ALK fusions (9 %), ROS1 fusions (3 %), and RET fusions (3 %). Eighty-two percent had already been treated with ≥ 3 prior lines.
Highly encouraging anti-tumor activity of Dato-DXd was observed in this heavily pretreated population. The ORR was 35 %, and responses lasted for a median of 9.5 months; this was consistent with the overall NSCLC population of the study [29]. Clinical activity emerged in the EGFR-mutated setting even after osimertinib failure. AEs were generally grade 1 and 2, with nausea and stomatitis being the most common events. The safety profile proved manageable and consistent with that observed in the overall NSCLC group [29]. Dato-DXd is being further evaluated in the TROPION-Lung05 trial in NSCLC patients with actionable genomic alterations after exhaustion of targeted agents and platinum-based chemotherapy options.
REFERENCES
- Stephens P et al., Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature 2004; 431(7008): 525-526
- Mazières J et al., Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 2013; 31(16): 1997-2003
- Li BT et al., Primary data from DESTINY-Lung01: a phase 2 trial of trastuzumab deruxtecan in patients with HER2-mutated metastatic non-small cell lung cancer. ESMO 2021, LBA45
- Cornelissen R et al., Efficacy and safety of poziotinib in treatment-naïve NSCLC harboring HER2 exon 20 mutations: a multinational phase 2 study (ZENITH20-4). ESMO 2021, LBA46
- Jatkoe T et al., Validation of companion diagnostics for the identification of patients with EGFR exon20ins NSCLC for amivantamab therapy. WCLC 2021, P24.14
- Leighl N et al., Amivantamab monotherapy and in combination with lazertinib in post-osimertinib EGFR-mutant NSCLC: analysis from the CHRYSALIS study. ESMO 2021, 1192M0
- Spira AI et al., Amivantamab in non-small cell lung cancer with MET exon 14 skipping mutation: initial results from CHRYSALIS. WCLC 2021, OA15.03
- Frampton GM et al., Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015; 5(8): 850-859
- Camidge DR et al., Telisotuzumab vedotin monotherapy in patients with previously treated c-Met+ advanced non-small cell lung cancer. WCLC, OA 15.04
- Riely GR et al., Activity and safety of mobocertinib (TAK-788) in previously treated non-small cell lung cancer with EGFR exon 20 insertion mutations from a phase I/II trial. Cancer Discov 2021; 11(7): 1688-1699
- Spira AI et al., Mobocertinib in EGFR exon 20 insertion-positive metastatic NSCLC patients with disease control on prior EGFR TKI therapy. ESMO 2021, OA15.01
- Jänne P et al., Phase 1 studies of DZD9008, an oral, selective EGFR/HER2 inhibitor in advanced NSCLC with EGFR exon20 insertion mutation. WCLC 2021, OA15.02
- Seto T et al., Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014; 15(11): 1236-1244
- Saito H et al., Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol 2019; 20(5): 625-635
- Nakagawa K et al., Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20(12): 1655-1669
- Piccirillo MC et al., Bevacizumab + erlotinib vs erlotinib alone as first-line treatment of patients with EGFR mutated advanced non squamous NSCLC. Final analysis of the multicenter, randomized, phase III BEVERLY trial. ESMO 2021, 12070
- Kenmotsu H et al., Primary results of a randomized phase II study of osimertinib plus bevacizumab versus osimertinib monotherapy for untreated patients with non-squamous non-small-cell lung cancer harboring EGFR mutations; WJOG9717L study. ESMO 2021, LBA44
- Girard N et al., Optimizing outcomes and treatment sequences in EGFR mutation-positive non-small-cell lung cancer: recent updates. Future Oncol 2019; 15(25): 2983-2997
- Popat S et al., Sequential afatinib and osimertinib in patients with advanced EGFRm+ NSCLC and acquired T790M: the real-world UpSwinG study. WCLC 2021, P51.05
- Kim ES et al., EGFR tyrosine kinase inhibitors for EGFR mutation-positive non-small-cell lung cancer: outcomes in Asian populations. Future Oncol 2021; 17(18): 2395-2408
- Hochmair MJ et al., Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: final analysis of the GioTag study. Future Oncol 2020; 16(34): 2799-2808
- Brueckl WM et al., Elderly patients treated with afatinib in clinical practice – final results of the GIDEON study in EGFR mutated NSCLC in Germany. ESMO 2021, 1230P
- Yang JCH et al., Afatinib for the treatment of NSCLC with uncommon EGFR mutations: an updated database of 1023 cases. ESMO 2021, 1212P
- Mito R et al., Clinical impact of TROP2 in non-small lung cancers and its correlation with abnormal p53 nuclear accumulation. Pathol Int 2020; 70(5): 287-294
- Inamura K et al., Association of tumor TROP2 expression with prognosis varies among lung cancer subtypes. Oncotarget 2017; 8(17): 28725-28735
- Jiang A et al., Expression and clinical significance of the Trop-2 gene in advanced non-small cell lung carcinoma. Oncol Lett 2013; 6(2): 375-380
- Meric-Bernstam F et al., TROPION-PanTumor 01: dose analysis of the TROP2-directed antibody-drug conjugate datopotamab deruxtecan for the treatment of advanced or metastatic non-small cell lung cancer. J Clin Oncol 39, 2021 (suppl 15; abstr 9058)
- Spira A et al., Datopotamab deruxtecan (Dato-DXd; DS-1062), a TROP2 ADC, in patients with advanced NSCLC: updated results of TROPION-PanTumor01 phase 1 study. WCLC 2020, OA03.03
- Garon EB et al., TROPION-PanTumor01: updated results from the NSCLC cohort of the phase 1 study of datopotamab deruxtecan in solid tumors. WCLC 2021, MA03.02
- Garon EB et al., Efficacy of datopotamab deruxtecan in patients with advanced/metastatic non-small cell lung cancer and actionable genomic alterations: preliminary results from the phase 1 TROPION-PanTumor01 study. ESMO 2021, LBA49
- Scagliotti GV et al., Addressing the unmet need in lung cancer: The potential of immuno-oncology. Cancer Treat Rev 2015; 41(6): 465-475
- Maione P et al., Overcoming resistance to targeted therapies in NSCLC: current approaches and clinical application. Ther Adv Med Oncol 2015; 7(5): 263-273
© 2021 Springer-Verlag GmbH, Impressum