New clinical insights in head and neck squamous cell carcinoma
Head and neck squamous cell carcinoma (HNSCC) was the sixth most common cancer in 2018, with more than 700,000 newly diagnosed cases per year and 350,000 cancer deaths worldwide [1]. Around 90 % of head and neck cancers are HNSCC, with oral cavity, oropharynx, hypopharynx, and larynx being the most commonly affected areas. The current standard of care (SOC) for locally advanced unresectable HNSCC is concurrent chemoradiotherapy (CRT) with high-dose cisplatin [2]. Pembrolizumab – a PD-1 immune checkpoint inhibitor – has been approved by the U.S. Food and Drug Administration (FDA) in June 2019 and by the European Medicines Agency (EMA) in November 2019 as first-line treatment for patients with metastatic or unresectable recurrent HNSCC either as monotherapy or in combination with platinum based chemotherapy [3, 4].
Primary results of the KEYNOTE-412 study
The randomized, double-blind, phase III KEYNOTE-412 (NCT03040999) study investigated the efficacy and safety of pembrolizumab versus placebo given concomitantly with CRT, followed by maintenance therapy with pembrolizumab or placebo in treatment-naïve patients with unresected locally advanced HNSCC (defined as T3-T4 [N0-N3] or any N2a-3 [T1-T4] larynx/hypopharynx/oral cavity/p16 negative oropharynx cancers and T4 or N3 p16 positive oropharynx cancer) [5]. At ESMO 2022, primary results of the KEYNOTE-412 trial were presented [6].
Overall, 804 patients (ECOG PS 0-1) were randomized 1:1 to receive either pembrolizumab (200 mg, IV) + cisplatin 100 mg/m2 every three weeks (Q3W) + CRT (70Gy/35F)) or placebo (Q3W) + CRT, followed by maintenance therapy with pembrolizumab or placebo for 14 cycles. Pembrolizumab or placebo priming was given one week before CRT. Stratification factors included radiotherapy regimen (accelerated fractionation [AFX] versus standard fractionation [SFX]), tumor site/p16 status (oropharynx [p16 positive versus negative] or larynx/hypopharynx/oral cavity) and disease stage (III versus IV).
Patients baseline characteristics were well balanced between both investigational groups. In both arms, most patients (around 85 %) showed a PD-L1 combined positive score (CPS) ≥ 1 and 36 % of them had CPS ≥ 20. Human papillomavirus (HPV) was found in 27 % of patients in the pembrolizumab arm versus 26 % in the placebo arm. After a median follow-up of 47.7 months, 86 % of patients in the pembrolizumab plus CRT arm and 88 % in the placebo plus CRT arm completed concurrent CRT and had ongoing maintenance therapy. In total, 210 patients (60 %) in the pembrolizumab arm and 223 (63 %) in the placebo arm completed the maintenance therapy at data cut-off. Of note, the cumulative cisplatin exposure of ≥200 mg/m2 was comparable in both arms (>87 %).
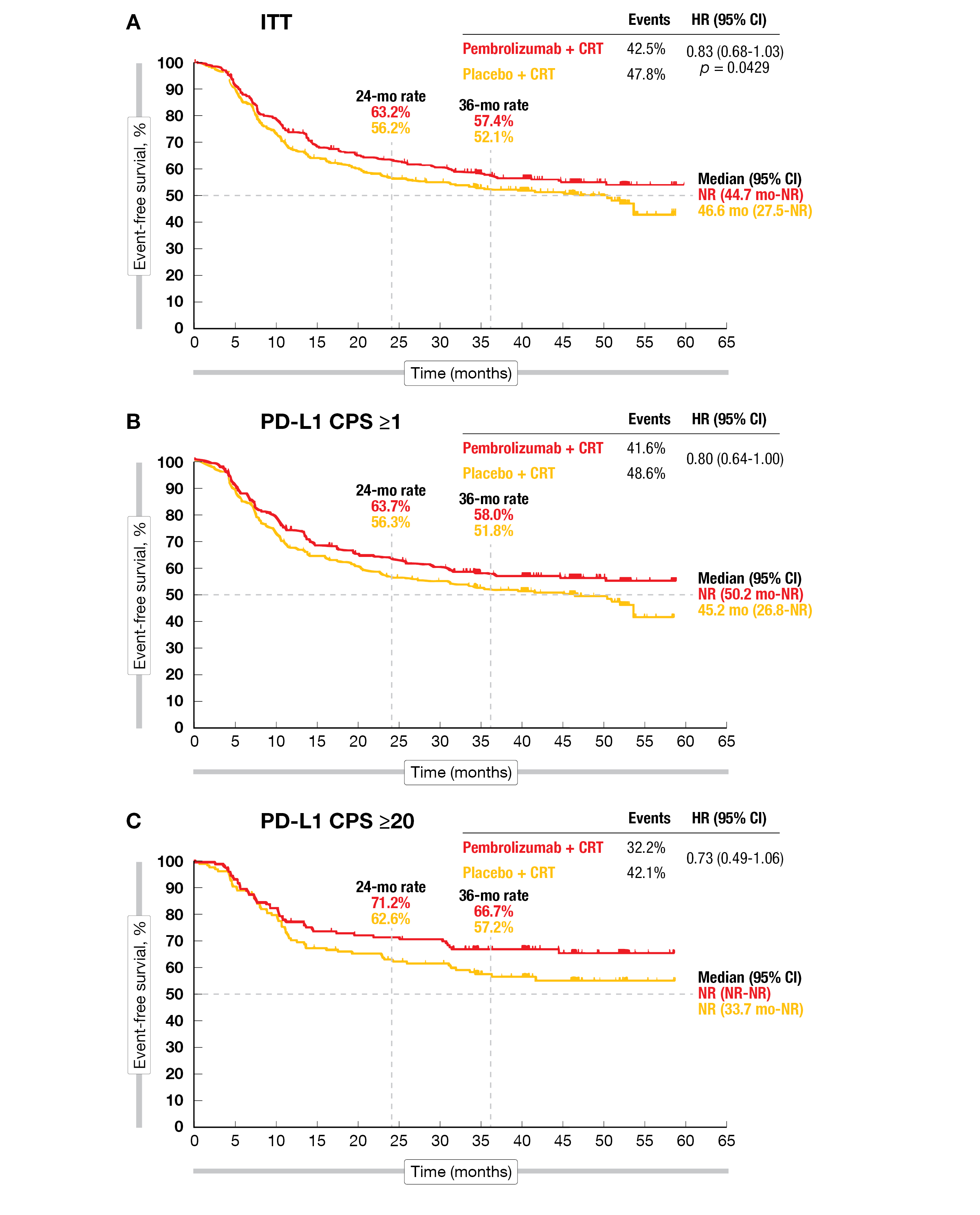
Event-free survival (EFS) – the primary endpoint – included death from any cause, progression according to RECIST v1.1 and pathologic proven relapse. A positive trend towards improved EFS was observed in favor of pembrolizumab plus CRT (NR vs 46.6 months with placebo; HR=0.83; 95 % CI, 0.68-1.03; p=0.0429), but the difference did not reach statistical significance. The 24-month EFS-rate was 63.2 % with pembrolizumab versus 56.2 % with placebo, while the 36-month rate was 57.4 % versus 52.1 %, respectively. Locoregional progressive disease (PD) was similar (13.2 % and 14.2 %) in both arms; however, distant PD was lower in the pembrolizumab arm (12.9 % versus 16.7 %). The non-significant benefit was observed in all prespecified subgroups analyzed except in the CPS < 1 group (HR=1.09; 95 % CI, 0.56-2.11). In a post-hoc analysis of the CPS ≥ 1 population, the 24-month EFS-rate was 63.7 % in the pembrolizumab arm versus 56.3 % in the placebo group, while the 36-month OS-rate achieved 71.4 % versus 70.2 %, respectively. Similar results were seen in the CPS ≥ 20 population, with a 24-month EFS-rate of 71.2 % in the pembrolizumab arm versus 62.6 % in the placebo group (Figure 1), and a 36-month OS-rate of 79.1 % versus 73.0 %, respectively. The overall survival (OS) was similar in both treatment arms (HR=0.90; 95 % CI, 0.71-1.15), but the median OS was not reached.
No new safety signals were reported. In total, 367 (92.2 %) patients in the pembrolizumab arm and 352 (88.4 %) in the comparative group experienced grade 3-5 adverse events (AEs), with four and six treatment-related deaths, respectively. In the pembrolizumab arm, 8.6 % of patients suffered from grade 3-5 immune-mediated AEs and infusion reactions compared with 2.3 % in the placebo arm.
The primary results of the KEYNOTE-412 study showed a favorable trend towards an improved EFS with first-line pembrolizumab plus CRT in locally advanced HNSCC patients although the difference did not reach statistical significance.
Figure 1: Event-free survival in the KEYNOTE-412 study in the ITT population (A), in patients with PD-L1 CPS ≥1 (B) and PD-L1 CPS ≥20 (C).
Long-term data of xevinapant in locally advanced HNSCC patients
Xevinapant (formerly known as Debio 1143) is an investigational first-in-class potent oral small-molecule inhibitor of apoptosis proteins (IAP) [7]. In preclinical studies, xevinapant restored the sensitivity of cancer cells to apoptosis and enhanced the effects of chemoradiotherapy (CRT) [8]. Efficacy and safety of xevinapant have been previously evaluated in a double-blind, multicenter, phase II study (NCT02022098) in 96 patients with previously untreated locally advanced HNSCC [9]. The locoregional control rate at 18 months after CRT, the primary endpoint, was significantly improved with xevinapant versus placebo (OR=2.74; 95 % CI, 1.15-6.53; p=0.0232) [10]. The 3-year progression-free survival (PFS), the key secondary endpoint, was also markedly improved with xevinapant (KM-estimate, 72 % versus 36 %; adjusted HR=0.33; 95 % CI, 0.17-0.67; p=0.0019) [10]. Long-term efficacy outcomes were presented at this year’s ESMO meeting [10].
High risk previously untreated locally advanced HNSCC patients were randomized 1:1 and stratified according to primary tumor site (oropharynx versus other), lymph node involvement (N0-N1 versus N2-N3), and HPV-16 status. Eligible patients received either xevinapant (200 mg/day, orally) or placebo once daily on days 1-14 of 21-day treatment cycles, for three cycles + CRT (cisplatin 100 mg/m2 on Day 2, Q3W for 3 cycles; intensity-modulated radiotherapy 70 Gy [2 Gy/day, 5 days/week for 7 weeks]).
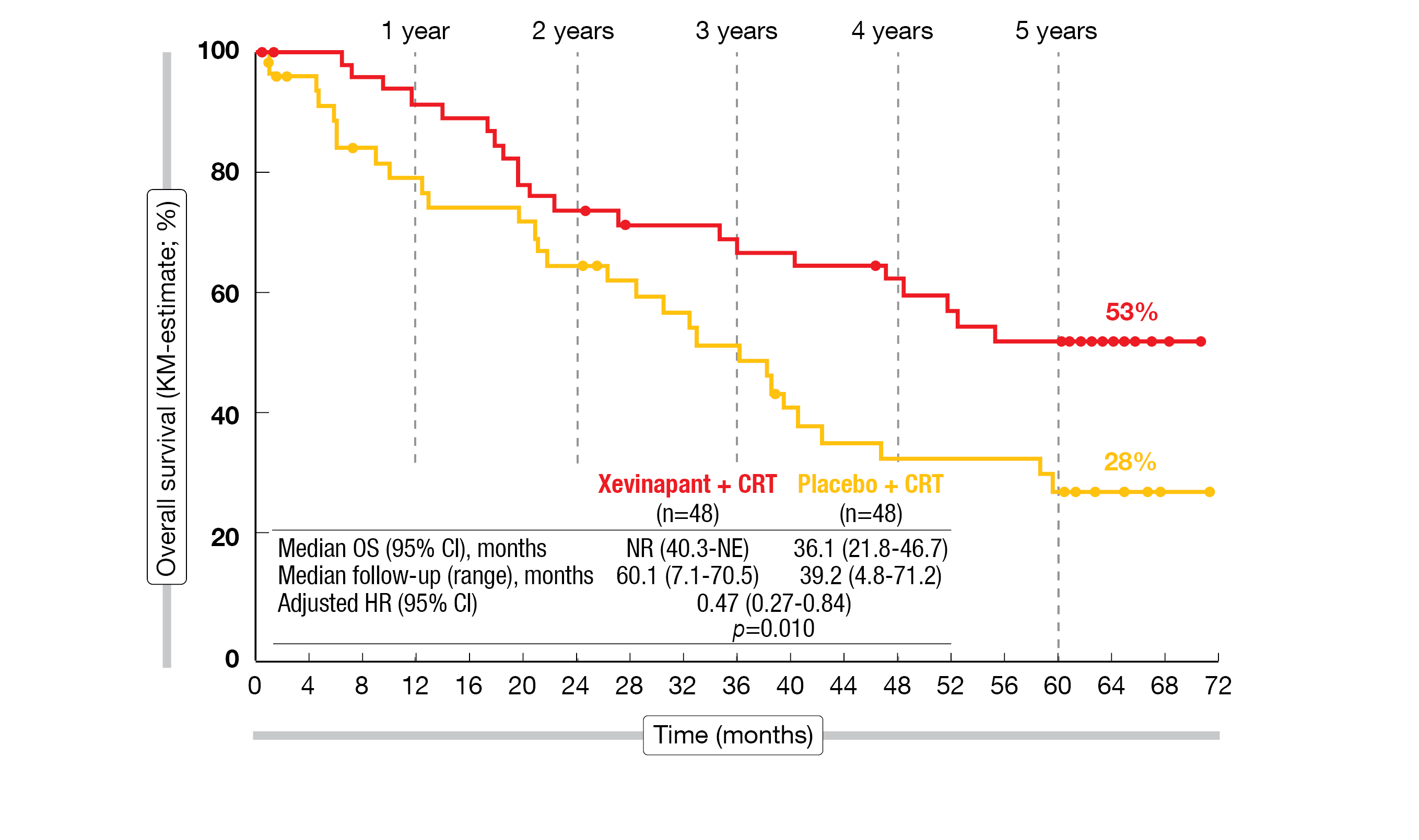
Between January 2016 and April 2017, 96 patients were followed-up for disease progression until July 2020 and survival data were collected until April 2022 (5 years after last patient randomization). The risk of death was more than halved in the xevinapant + CRT arm versus the placebo + CRT arm (adjusted HR=0.47; 95 % CI, 0.27-0.84; p=0.0101). The median OS was not reached in the xevinapant arm versus 36.1 months (95 % CI, 21.8-46.7) in the placebo arm. For long-term OS, the median follow-up was 60.1 months in the xevinapant arm and 39.2 months in the placebo group. Xevinapant + CRT prolonged OS compared to placebo + CRT, with a 53 % (95 % CI, 37-66) OS rate after five years compared to 28 % (95 % CI, 15-42) with placebo (Figure 2).
The duration of response (DoR) was prolonged with xevinapant plus CRT. The risk of death or disease progression after initial response was markedly reduced by 79 % in the xevinapant arm versus placebo (DoR KM-estimate, 79 % vs 36 %; adjusted HR=0.21; 95 % CI, 0.08-0.54; p=0.0011).
The safety profile, including late-onset AEs, was similar in both investigational arms. The most frequent grade ≥ 3 AEs were dry mouth and dysphagia in the xevinapant group, compared to anemia and dysphagia in the placebo group. In total, eight patients in the xevinapant and seven in the placebo arm had to discontinue the study treatment prematurely.
When added to the standard of care CRT, xevinapant showed a significant OS-improvement in patients with locally advanced HNSCC. Based on these results, xevinapant plus CRT is currently being further investigated in the ongoing phase III TrilynX study (NCT04459715).
Figure 2: 5-year OS of xevinapant + CRT versus placebo + CRT in unresected locally advanced HNSCC.
KEYNOTE-048: first-line pembrolizumab in R/M HNSCC long term follow-up
The phase III KEYNOTE-048 trial (NCT02358031) investigated pembrolizumab monotherapy and pembrolizumab plus chemotherapy versus cetuximab plus chemotherapy (EXTREME) in previously untreated recurrent or metastatic (R/M) HNSCC [11]. Previous analyses of KEYNOTE-048 showed a significant improvement of the median OS with pembrolizumab monotherapy or pembrolizumab plus chemotherapy in pre-specified subgroups, compared to cetuximab plus chemotherapy, with comparable safety. Five-year results from KEYNOTE-048 were presented at ESMO 2022 [12].
Eligible participants with R/M HNSCC of the oropharynx, oral cavity, hypopharynx, or larynx that was not curable by local therapy were randomly assigned 1:1:1 to either receive pembrolizumab (200 mg, Q3W for up to 35 cycles), pembrolizumab plus chemotherapy (carboplatin AUC 5 or cisplatin 100 mg/m2 + 5-FU 1000 mg/m2/day for 4 days for 6 cycles (Q3W)), or cetuximab (400 mg/m2 loading dose, then 250 mg/m2 per week) plus chemotherapy given until PD, unacceptable toxicity, six cycles (chemotherapy), or 24 months (pembrolizumab). Stratification factors included ECOG (0 versus 1), p16 status in oropharynx, and PD-L1 expression (Tumor Proportion Score (TPS) ≥ 50 % versus < 50 %) [11].
After a median follow-up of 69.2 months, pembrolizumab monotherapy showed an improved 5-year OS versus EXTREME in the CPS ≥ 20 (19.9 % vs 7.4 %), CPS ≥ 1 (15.4 % vs 5.5 %) and total population (14.4 % vs 6.5 %) [12]. The ORR for pembrolizumab versus EXTREME was 16.9 % versus 36.0 %, and the DoR 22.6 months versus 4.5 months, respectively.
Similarly, pembrolizumab plus chemotherapy improved the 5-year OS versus EXTREME in the CPS ≥ 20 population (23.9 % versus 6.4 %), CPS ≥ 1 population (18.2 % versus 4.3 %), and in the total population (16.0 % versus 5.2 %). The ORR was 37.0 % versus 36.3 % and the median DoR reached 6.7 months versus 4.3 months, respectively.
The safety profile was consistent to previous reports.
The authors concluded that, even after five years of follow-up, first-line pembrolizumab therapy continued to show a clinically meaningful benefit for patients with R/M HNSCC.
KEYNOTE-B10: pembrolizumab plus carboplatin/paclitaxel as first-line 5-FU-free therapy option in R/M HNSCC
Based on the positive outcomes of the pivotal KEYNOTE-048 trial, pembrolizumab combined with platinum plus fluorouracil (5-FU) has been approved as 1L therapy for R/M HNSCC [13]. However, alternatives to 5-FU are needed to overcome toxicities, costs and complications related to the continuous 4-day infusion of this drug [14]. The combination of cisplatin plus paclitaxel was shown to be as effective as cisplatin plus 5-FU in patients with R/M HNSCC [15]. Thus, the goal of the global, open-label, phase IV KEYNOTE-B10 study (NCT04489888) was to evaluate the antitumoral activity and safety of pembrolizumab combined with carboplatin and paclitaxel as first-line treatment in patients with R/M HNSCC. The primary analysis of this ongoing study was presented at ESMO 2022 [16].
Eligible patients with previously untreated R/M HNSCC of oral cavity, oropharynx, larynx, or hypopharynx received pembrolizumab (200 mg, Q3W for ≤ 35 cycles) plus paclitaxel (investigators choice for 6 cycles) and carboplatin (AUC 5 mg/mL/min, Q3W for 6 cycles). The primary study endpoint is ORR per RECIST v1.1 assessed by blinded independent central review (BICR), the secondary endpoints include DoR and PFS according to RECIST v1.1 by BICR, OS as well as safety and tolerability. The presented analysis enclosed 92 treated patients (of 100 planned patients); among them, 41 participants were still on treatment after a median follow-up of 8.2 months.
The median age of the patient population was 64 years, most of the patients were male (82.6 %) and white (87.0 %); three quarters of the patients had a paclitaxel dose of 175 mg/m2 Q3W, 18.5 % had a PD-L1 expression CPS < 1, 66.3 % had distant metastasis, and 21.7 % of the oropharynx patients were p16 positive.
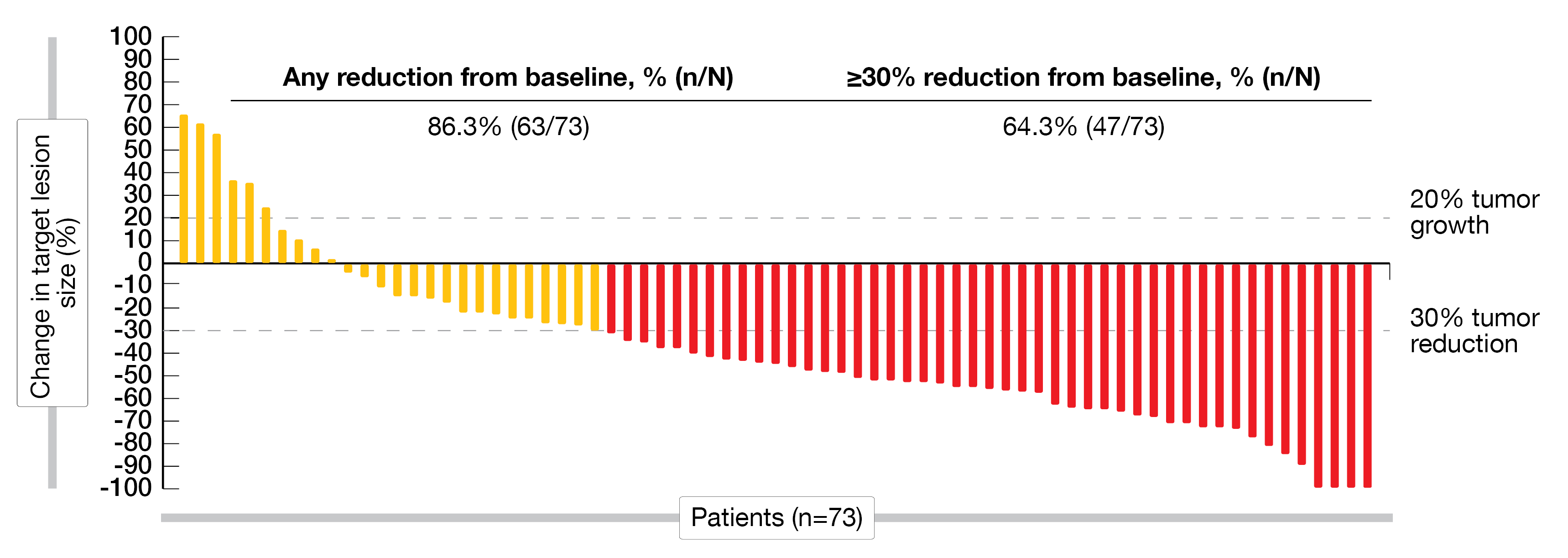
Out of 82 patients included in the efficacy analysis, 42.7 % had an objective response, with four patients showing a complete response (CR) and 31 a partial response (PR); moreover, 24 patients had a stable disease (SD), while 15 patients had a progressive disease (PD). The disease control rate (DCR) was 58.5 % and the median DoR reached 5.5 months. Most patients showed a reduction from baseline for target lesions (Figure 3).
Nearly all patients (95.7 %) reported treatment related adverse events (TRAEs) any grade. Overall, 70.7 % experienced grade 3-5 TRAEs, including two deaths (2.2 %) due to TRAEs (sepsis and hypersensitivity). The most reported TRAEs with ≥ 10 % incidence were decreased neutrophil count, anemia, and fatigue. No new immune-mediated AEs and infusion reactions were detected.
This first global, prospective trial of pembrolizumab plus carboplatin and paclitaxel demonstrated antitumor activity in first-line R/M HNSCC independently of PD-L1 status with a manageable safety profile. The results of the KEYNOTE-B10 study may suggest this 5-FU-free chemotherapy combination with pembrolizumab as an alternative to the current SOC chemotherapy regime.
Figure 3: Waterfall-plot indicating the best percentage of change from baseline for target lesions.
KEYNOTE-040 study: long-term follow-up
The open-label, phase III KEYNOTE-040 study (NCT02252042) compared the efficacy and safety of pembrolizumab with those of an investigator’s SOC choice of methotrexate (40 mg/m2, QW) or docetaxel (75 mg/m2, Q3W) or cetuximab (250 mg/m2, QW) in patients with R/M HNSCC who progressed during or after platinum-based chemotherapy within 3-6 months of multimodal therapy. Six-year follow up data of the KEYNOTE-040 trial were presented at ESMO 2022 [17].
After a median follow-up of 74.8 months, the 6-year survival rate accounted for 6.5 % in the pembrolizumab arm versus SOC regime with 2.4 %. For PD-L1 positive patients (CPS ≥ 1), the risk reduction of death with pembrolizumab was 28 % (8.7 versus 7.1 months; HR=0.72; 95 % CI, 0.59-0.89), with a 6-year OS rate of 7.1 % versus 2.1 %, respectively. For patients with TPS ≥ 50 %, the risk reduction of death remained at 38 % (11.6 months versus 7.9 months; HR=0.62; 95 % CI, 0.43-0.90), with a 6-year OS rate of 8.9 % versus 6.3 %, respectively.
After a follow-up of six years, pembrolizumab continued to show an OS benefit compared to SOC in R/M HNSCC patients. To note, an increased benefit was observed with increasing PD-L1 expression. Overall, these data further support the use of pembrolizumab as second-line treatment in appropriate patients with R/M HNSCC.
REFERENCES
- Ortiz-Cuaran, S, et al., Precision Medicine Approaches to Overcome Resistance to Therapy in Head and Neck Cancers. Front Oncol 2021; 11: 614332.
- Machiels, JP, et al., Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020; 31(11): 1462-1475.
- FDA approves pembrolizumab for first-line treatment of head and neck squamous cell carcinoma. 2019, last accessed on October 30, 2022]; Available from: https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-keytruda-ii-65_en.pdf.
- SmPC Keytruda. 2019, last accessed on October 30, 2022]; Available from: https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-keytruda-ii-65_en.pdf.
- Machiels, JP, et al., Pembrolizumab given concomitantly with chemoradiation and as maintenance therapy for locally advanced head and neck squamous cell carcinoma: KEYNOTE-412. Future Oncol 2020; 16(18): 1235-1243.
- Machiels J., et al., Primary results of the phase III KEYNOTE-412 study: Pembrolizumab (pembro) with chemoradiation therapy (CRT) vs placebo plus CRT for locally advanced (LA) head and neck squamous cell carcinoma (HNSCC). Ann Oncol 2022; 33(suppl 7; Abstr LBA5).
- Le Tourneau, C, et al., Phase I Trial of Debio 1143, an Antagonist of Inhibitor of Apoptosis Proteins, Combined with Cisplatin Chemoradiotherapy in Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck. Clin Cancer Res 2020; 26(24): 6429-6436.
- Matzinger, O, et al., The radiosensitizing activity of the SMAC-mimetic, Debio 1143, is TNFα-mediated in head and neck squamous cell carcinoma. Radiother Oncol 2015; 116(3): 495-503.
- Sun, XS, et al., Debio 1143 and high-dose cisplatin chemoradiotherapy in high-risk locoregionally advanced squamous cell carcinoma of the head and neck: a double-blind, multicentre, randomised, phase 2 study. Lancet Oncol 2020; 21(9): 1173-1187.
- Bourhis J., et al., 5-year overall survival (OS) in patients (pts) with locally advanced squamous cell carcinoma of the head and neck (LA SCCHN) treated with xevinapant + chemoradiotherapy (CRT) vs placebo + CRT in a randomized, phase II study. Ann Oncol 2022; 33(suppl 7; Abstr LBA33).
- Burtness, B, et al., Pembrolizumab Alone or With Chemotherapy for Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma in KEYNOTE-048: Subgroup Analysis by Programmed Death Ligand-1 Combined Positive Score. J Clin Oncol 2022; 40(21): 2321-2332.
- Tahara M., et al., Pembrolizumab with or without chemotherapy for first-line treatment of recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): 5-year results from KEYNOTE-048. Ann Oncol 2022; 33(suppl 7; Abstr 659MO).
- FDA approves pembrolizumab for first-line treatment of head and neck squamous cell carcinoma. 2019, [cited last accessed on October 30, 2022; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-first-line-treatment-head-and-neck-squamous-cell-carcinoma.
- Guigay, J, et al., The Evolving Role of Taxanes in Combination With Cetuximab for the Treatment of Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck: Evidence, Advantages, and Future Directions. Front Oncol 2019; 9: 668.
- Gibson, MK, et al., Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2005; 23(15): 3562-3567.
- Dzienis M., et al., Pembrolizumab (pembro) + carboplatin (carbo) + paclitaxel (pacli) as first-line (1L) therapy in recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): Phase VI KEYNOTE-B10 study. Ann Oncol 2022; 33(suppl 7; Abstr 651O).
- Soulieres M., et al., Pembrolizumab (pembro) vs standard-of-care (SOC) in previously treated recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): 6-year follow-up of KEYNOTE-040. Ann Oncol 2022; 33(suppl 7; Abstr 658MO).
© 2022 Springer-Verlag GmbH, Impressum
More posts
Findings obtained with immunotherapeutic combination approaches
Approximately of 95 % metastatic colorectal cancer (mCRC) cases are characterized by microsatellite stability (MSS) [1]. In this group, traditional immune-based treatment has consistently failed, giving rise to an unmet medical need regarding effective treatment options in the setting of chemotherapy-refractory MSS mCRC [2-5].
Marwan G. Fakih
ASCO-GI 2025 - Marwan G. Fakih Marwan G. Fakih addresses the urgent need for new the ...
Meghan C. Thompson
ASH 2024 - Meghan C. Thompson Meghan C. Thompson discusses outcomes in patients with ...
Mantle cell lymphoma: optimizing responses in treatment-naïve and difficult-to-treat patients
The three-arm, randomized, phase III TRIANGLE trial has set a new first-line standard in younger patients with mantle cell lymphoma (MCL), showing that the addition of ibrutinib to standard immunochemotherapy improves efficacy [1]. Previously untreated patients aged 18-65 years who were eligible for autologous stem cell transplantation (ASCT) were randomized to either induction treatment with R-CHOP and R-DHAP followed by ASCT (group A; n = 288) or one of two experimental arms: In group A+I (n = 292), ibrutinib was added to R-CHOP and was administered as fixed-duration maintenance for two years after ASCT.
Advancing care in Waldenström macroglobulinemia: Clinical and real-world perspectives
Recent advances have transformed the management of Waldenström macroglobulinemia (WM), particularly with the advent of targeted therapies such as Bruton tyrosine kinase (BTK) inhibitors. These innovations have addressed longstanding challenges including resistance, intolerance, and the need for personalized approaches (enabling therapy also for those deemed unfit for chemotherapy).
Follicular lymphoma: Efficacy and safety updates
In the context of follicular lymphoma treatment, there is a clear unmet need for patients who have experienced early progression (progression within 24 months of front-line therapy, POD24), as well as those who are refractory to treatment and present with other high-risk features. At ASH 2024, promising efficacy and safety data were reported for the five molecules nemtabrutinib, zanubrutinib, BGB-16673, epcoritamab, and mosunetuzumab [1-6].