Immunostimulation as a promising approach in SCLC
IMPULSE
There is a high unmet medical need regarding extensive-disease small-cell lung cancer (SCLC) that shows poor outcomes with median OS of 9 to 11 months. First-line chemotherapy usually evokes marked responses, but responders typically experience only limited periods of disease control.
Based on the hypothesis that activation of the immune system might prolong disease stability in these patients, thus ultimately affecting their survival, Thomas et al. assessed the activity of the toll-like receptor 9 (TLR9) agonist lefitolimod [1]. Lefitolimod initiates immune surveillance by broad enhancement of the innate and adaptive immune system via multiple pathways, taking advantage of the decreased tumour burden and released tumour antigens during chemotherapy [2–4].
The exploratory, randomised, controlled, phase II IMPULSE study took place at 41 centres in Belgium, Austria, Germany and Spain. Patients with extensive-disease SCLC who had already developed PR or CR after four cycles of platinum-based induction chemotherapy were enrolled. They were randomised in a 3:2 ratio either to the experimental group (n = 61) that was treated with lefitolimod plus platinum-based chemotherapy (5th/ 6th cycle) followed by lefitolimod maintenance, or to the control group (n = 41). Here, patients only received the 5th/ 6th cycle of chemotherapy followed by subsequent treatment according to local practice. Lefitolimod was administered at a dose of 60 mg subcutaneously twice weekly. OS in the intent-to-treat (ITT) population was defined as the primary endpoint of the IMPULSE study.
Confirmation of the mode of action
A selected secondary endpoint of the trial consisted in the standardised detection of pharmacodynamic markers (i.e., activation of monocytes and secretion of the chemokine IP-10) to confirm the mode of action of lefitolimod. Monocytes and IP-10 were assessed in a comparative manner before the initiation of treatment and at least 4 weeks thereafter. Indeed, significant increases of CD169-positive monocyte counts and IP-10 levels occurred as expected. IMPULSE demonstrated limited add-on toxicity of lefitolimod in combination with chemotherapy. Cough and headache preponderated in the experimental arm compared to the control arm. Grade 3 AEs occurred only infrequently, and no grade 4 or 5 AEs were reported.
Although the OS analysis of the ITT population revealed no significant difference in survival (279.0 vs. 272.0 days with the lefitolimod-based regimen and chemotherapy only, respectively; HR, 1.27; p = 0.53), there were signals of activity of lefitolimod in certain subgroups according to pre-planned analyses. Patients with reported chronic obstructive pulmonary disease (COPD) experienced a 46 % reduction in their mortality risk (316.0 vs. 246.0 days; HR, 0.54).
Activity in the presence of low activated B cell counts
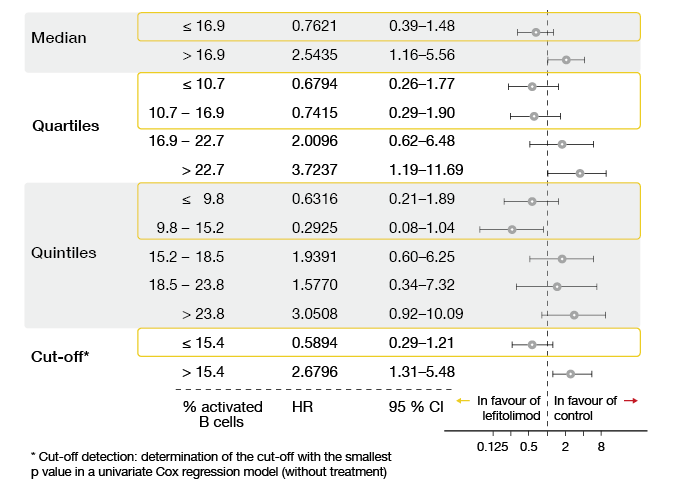
Interesting results were obtained for the population with low numbers of activated B cells at baseline. This cohort comprised 38 individuals, 23 of whom received lefitolimod. Median OS was 284.0 vs. 231.5 days with lefitolimod and chemotherapy only for these patients (HR, 0.59). Activated B cells were defined as the CD86-positive proportion of CD19-positive B cells, with a cut-off at 15.4 %. The predictive value of low activated B cell counts persisted across different analyses (i.e., median, quartiles, quintiles, delineated cut-off; Figure).
This phenomenon might be due to suppression of the lefitolimod-triggered anti-tumour response by activated/ regulatory B cells, which implies that low numbers of these cells facilitate the full effect of lefitolimod treatment. Next steps include the validation of lefitolimod in a patient population with low counts of activated B cells.
Figure: Overall survival in patients with low counts of activated B cells across different analyses
References
- Thomas M et al., Top-line data from the randomized phase 2 IMPULSE study in small-cell lung cancer (SCLC): immunotherapeutic maintenance treatment with lefitolimod. ESMO 2017, abstract 1527O
- Kapp K et al., Genuine immunomodulation with dSLIM. Mol Ther Nucleic Acids 2014; 3: e710
- Schmidt M et al., Design and structural requirements of the potent and safe TLR-9 agonistic immunomodulator MGN1703. Nucleic Acid Ther 2015; 25(3): 130-134
- Wittig B et al., MGN1703, an immunomodulator and toll-like receptor 9 (TLR-9) agonist: from bench to bedside. Crit Rev Oncol Hematol 2015; 94(1): 31-44
More posts
ALK-positive NSCLC: updates on crizotinib and alectinib
PROFILE 1014 was the first study to define the role of the ALK inhibitor crizotinib in the first-line treatment of patients with ALK-positive lung cancer. It compared crizotinib 250 mg twice daily (n = 172) with pemetrexed plus cisplatin (n = 171) in patients with ALK-positive, locally advanced, recurrent or metastatic non-squamous NSCLC in the first-line setting.
Characteristics and outcomes for SCLC arising from transformation
A low but significant proportion of EGFR-mutant adenocarcinomas transforms to SCLC at the time of acquisition of resistance to EGFR TKI therapy. Moreover, cases of de novo SCLC harbouring EGFR mutations have been reported. As the clinical characteristics and clinical course of SCLC-transformed EGFR-mutant lung cancer are largely unknown, Marcoux et al. retrospectively reviewed the records of 16 patients with EGFR-mutant SCLC treated between 2006 and 2017.
Reaching unprecedented outcome dimensions in malignant mesothelioma
Malignant pleural mesothelioma (MPM) is a rare but aggressive cancer with poor prognosis. While combination chemotherapy with platinum and pemetrexed with or without bevacizumab is a standard in first-line treatment, no approved second-line strategies have been established to date. Gemcitabine or vinorelbine are often used in this situation, but these only show limited activity.
Interview: “Survival is the result of multiple treatment lines”
FLAURA is a positive trial, as its results favour osimertinib over gefitinib and erlotinib. Now we have to consider this among the multiple options that are available for the first-line treatment of EGFR-mutant lung cancer. Besides osimertinib, there are the first-generation TKIs erlotinib and gefitinib and the second-generation TKI afatinib, but maybe sometime soon also dacomitinib, for which data were presented at the last ASCO Meeting.
EGFR-mutant lung cancer: sequencing as a major topic in light of new data
The first-generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) erlotinib and gefitinib as well as the second-generation EGFR TKI afatinib are the recommended first-line options for patients with EGFR-mutant NSCLC. Regardless of the extent of initial response, however, more than 60 % of patients develop the T790M resistance mutation.
Randomised findings on CT-based follow-up after resection of early NSCLC
Regarding the optimal follow-up after surgery for early-stage NSCLC, the ESMO guidelines recommend patient surveillance every six months for 2-3 years with visits including history, physical examination and preferably contrast-enhanced spiral chest CT at 12 and 24 months. Thereafter, annual visits including history, physical examination and chest CT should be performed to detect second primary tumours (SPCs).