Improved median PFS for patients with late-stage non-squamous NSCLC treated with first-line sintilimab plus conventional chemotherapy regime
Orient-11 evaluated the efficacy and safety of the addition of sintilimab, an anti-PD-1 antibody, to conventional chemotherapy combination of platinum and pemetrexed as first-line therapy in patients with non-squamous non-small cell lung cancer (nsq NSCLC). The trial was conducted in China, and the inclusion criteria were the diagnosis of untreated locally advanced or metastatic, Stage IIIB/C or IV nsq NSCLC with ECOG performance status of 0 or 1 and absence of sensitive EGFR or ALK genetic rearrangements.
In total, 397 patients were randomized in a 2-to-1-ratio to receive treatment with either sintilimab or placebo in combination with chemotherapy of pemetrexed and platinum (either cisplatin or carboplatin) every three weeks for four cycles, followed by maintenance treatment with pemetrexed plus either sintilimab or placebo for up to 24 months. Importantly, patients in the placebo group could crossover within the study, to maintenance treatment with sintilimab monotherapy after central confirmation of progression, for up to 24 months. Patients were stratified based on gender, type of platinum agent and PD-L1 expression (tumor proportion score (TPS) <1% vs ≥1%).
Baseline characteristics of patients in both treatments were similar with a higher proportion of patients being male (~75%), with Stage IV disease (~90%) and on carboplatin regimen (~75%). Interestingly, this trial had a higher percentage of never smokers in both treatment groups (~35%) compared to earlier studies in similar indications.
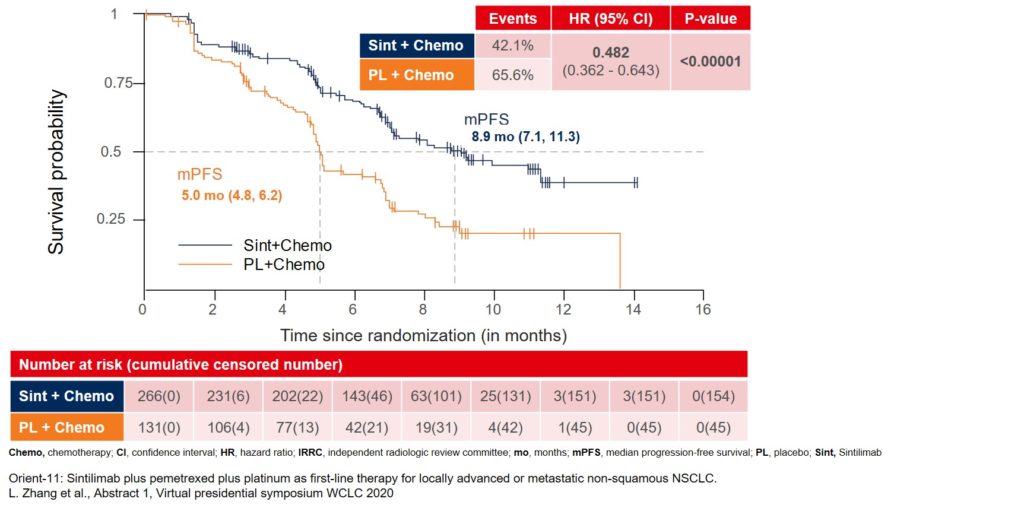
The primary endpoint of the trial was met. Median progression-free survival (mPFS) assessed by an independent radiologic review committee (IRRC) improved to 8.9 months in the sintilimab group as compared to 5 months in the placebo group (Figure 1).
Figure 1: Median PFS assessed by IRRC as the primary endpoint for the ORIENT-11 study
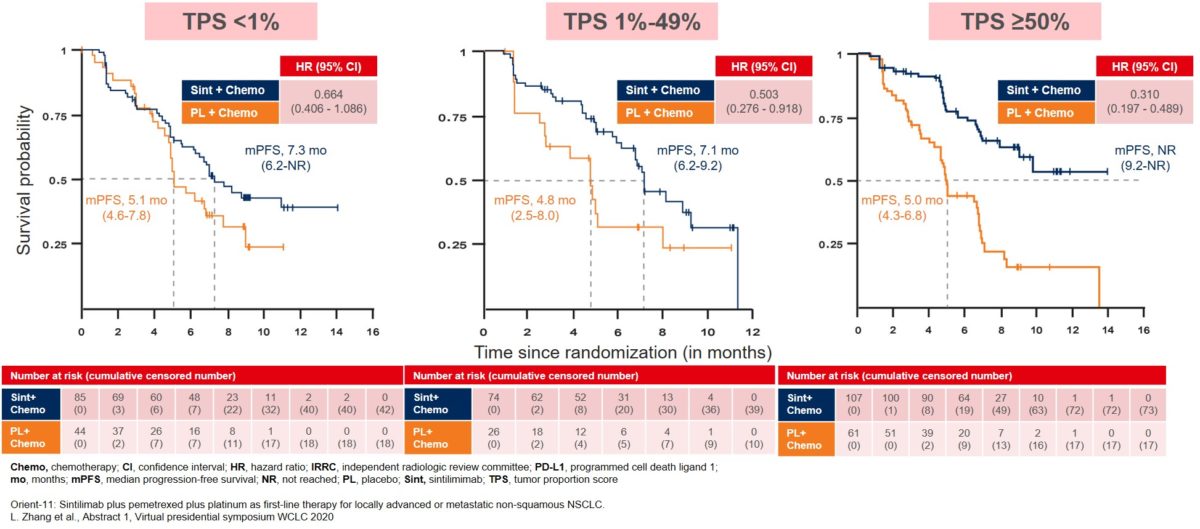
The mPFS improvement was consistent across all patient subgroups, and especially in the subgroup expressing ≥50% PD-L1 TPS with mPFS not reached until the analysis (Figure 2). The patients in the other two PD-L1 TPS subgroups also showed improvement in their mPFS, though to a lesser extent of around two months, with mPFS for TPS <1% at 7.3 months and TPS 1-49% at 7.1 months for the sintilimab treatment group (Figure 2).
Figure 2: Median PFS in patient subgroups based on PD-L1 tumor proportion score (TPS)
The addition of sintilimab to chemotherapy demonstrated improvements in secondary efficacy endpoints as well. Overall survival (OS) data was not yet fully mature, with medians not reached at the time of data cut-off for either treatment arms, but showing a positive trend for sintilimab and chemotherapy combination regimen (6-month overall survival: 89.6% vs 80.4%, HR 0.609, 95% CI 0.400 – 0.926; p = 0.01921) with the median follow-up of 8.9 months. The combination arm also yielded a higher objective response rate (51.9% vs 29.8%), longer duration of response (not reached vs 5.5 months) and shorter time to response (1.5 vs 2.6 months) as compared to the placebo arm.
The overall safety profiles of both treatment groups were similar with anemia, neutropenia and leukopenia being the most common adverse events reported; the typical toxicity profile of pemetrexed plus platinum regimen. The proportion of immune-related AEs (irAEs) was predominantly higher in the treatment group with sintilimab as compared to the placebo group (43.25% vs 36.6%) with an increased incidence of hypothyroidism, rash, and immune-mediated pneumonitis. These increased irAEs were mostly low grade with incidences of high-grade irAEs (3-5) similar between the two groups (5.6% vs 6.1%).
In conclusion, the interim analysis of Orient-11 trial met its primary endpoint, and the data appears comparable to other chemoimmunotherapy studies, including KEYNOTE-189, which studied the addition of pembrolizumab to platinum plus pemetrexed regimen. Keeping in mind the caveats associated with cross-trial comparisons, longer follow-up from Orient-11 will be required to compare OS data between the trials.
More posts
Interview: Lung cancer screening: hurdles in daily routine and in the research laboratory
Interview: Lung cancer screening: hurdles in daily routine and in the research laboratory