Continuous BTK inhibition and combination regimens: present and future
Agents of outstanding efficacy
Inhibitors of Bruton tyrosine kinase (BTK) have changed the standards of care in the setting of chronic lymphocytic leukemia (CLL). However, they do not eliminate leukemia, and undetectable residual disease is only very rarely achieved. Continuous treatment is therefore the standard approach, with the covalent BTK inhibitors ibrutinib, acalabrutinib and zanubrutinib being in widespread use as front-line single agents. Also, responses to BTK inhibition tend to deepen over time [1].
Kerry A. Rogers, MD, James Cancer Hospital and Solove Research Institute, Columbus, Ohio, USA, pointed out that these are highly effective therapies, even in the high-risk setting. The NCCN guidelines for CLL/SLL list acalabrutinib ± obinutuzumab and zanubrutinib as preferred first-line options, along with venetoclax/obinutuzumab, for patients with and without del(17p)/TP53 mutations [2]. The outstanding efficacy of BTK inhibitors is demonstrated by trials such as the randomized E1912 study in which ibrutinib/rituximab outperformed the most effective chemoimmunotherapy regarding overall survival, reducing mortality by more than 50 % (HR, 0.47; p = 0.018) [3]. In the RESONATE-2 trial, ibrutinib monotherapy, as compared to chlorambucil, gave rise to a sustained significant progression-free survival (PFS) advantage, with 7-year PFS rates of 59 % vs. 9 % [1].
Outcome improvement in patients with TP53 aberration
Importantly, BTK inhibitor treatment confers significant benefits in TP53-disrupted CLL. In the Alliance A041202 trial, ibrutinib ± rituximab induced superior PFS compared to bendamustine/rituximab (BR), which was independent of the presence of TP53 abnormalities [4]. Fixed-duration treatment with venetoclax/obinutuzumab, on the other hand, did not abrogate the adverse effect of TP53 aberrations, as was shown by the CLL14 study [5]. Dr. Rogers pointed out that these findings support continuous BTK inhibitor therapy as the preferred option for patients with TP53-disrupted CLL. Nevertheless, according to real-world data, the presence of del(17p) still affects the overall survival obtained with first-line ibrutinib, although these outcomes are certainly improved compared to the previous treatment standard of chemoimmunotherapy [6].
Another advantage of BTK inhibitors results from the possibility of combinations with anti-CD20 monoclonal antibodies. While BTK inhibition is continuous, anti-CD20 therapy is commonly restricted to 6 cycles. The choice of agents appears to make a difference. Rituximab plus ibrutinib did not improve PFS over ibrutinib alone in the Alliance A041202 trial [4], whereas obinutuzumab plus acalabrutinib was shown to increase PFS compared to acalabrutinib monotherapy in the ELEVATE-TN study (HR, 0.51; p = 0.0259) [7]. Moreover, the addition of obinutuzumab to acalabrutinib improved the undetectable MRD (uMRD) rate vs. acalabrutinib alone (42 % vs. 2 %), which raises the question of treatment discontinuation. However, as Dr. Rogers noted, further research is called for in this respect.
Specific toxicity profiles
Randomized comparisons across covalent BTK inhibitors in pretreated patients have revealed diverse results. While the ELEVATE-RR study did not show a significant PFS difference between ibrutinib and acalabrutinib [8], zanubrutinib prolonged PFS vs. ibrutinib in the ALPINE trial (HR, 0.65) [9]. In both ELEVATE-RR and ALPINE, the rates of atrial fibrillation/flutter were higher with ibrutinib than with the next-generation BTK inhibitors acalabrutinib and zanubrutinib. Hypertension occurred less commonly with acalabrutinib vs. ibrutinib. However, not all adverse events (AEs) are reduced with the newer drugs; for bleeding events, the reduction is unclear, and specific AEs such as headache for acalabrutinib and neutropenia for zanubrutinib have been reported. Nevertheless, the availability of several agents allows for some tailoring of treatment and for switching in the setting of intolerance, thus enabling some patients to continue BTK inhibitor therapy after the emergence of unacceptable AEs.
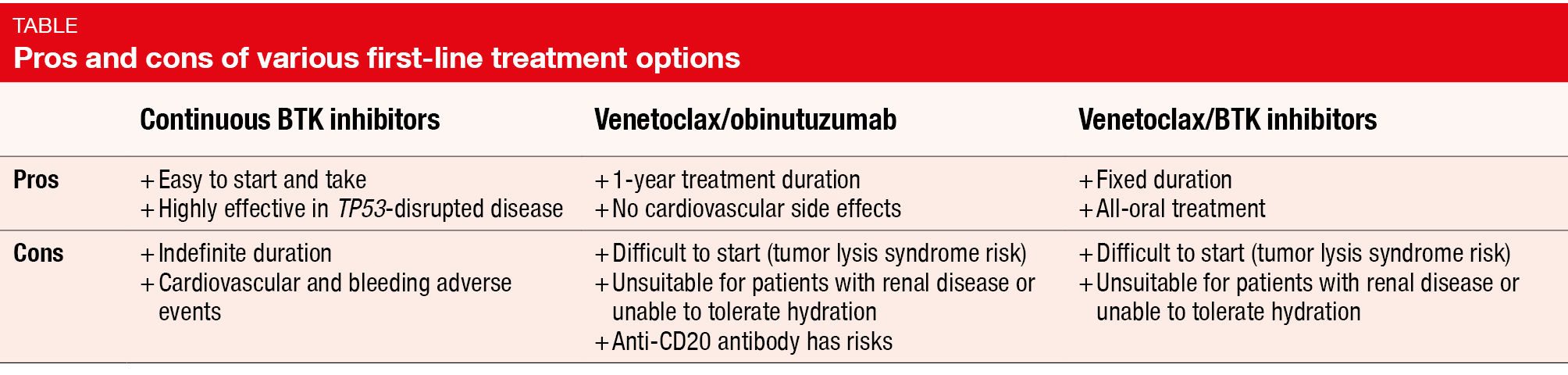
In addition, BTK inhibitors represent a convenient therapy for both patients and physicians. Oral daily regimens are easy to administer and can be used in a wide variety of settings. While venetoclax confers a considerable risk of tumor lysis syndrome, the initiation of BTK inhibition requires only limited monitoring (Table). Generally, BTK inhibitors are well-tolerated drugs, and many patients are hardly impaired with regard to their daily activities. Current questions in the setting of BTK inhibition relate to the ideal timing of discontinuation of continuous monotherapy and the definition of differences between covalent BTK inhibitors in terms of efficacy, toxicity, and resistance. Another issue is optimal sequencing of the next CLL treatment(s) in case of progression.
BTK/BCL-2 inhibitor combinations
Nitin Jain, MD, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA, presented clinical trials exploring BTK-inhibitor–based doublets and triplets in previously untreated patients with CLL. The all-oral combination of BTK and BCL-2 inhibitors potentially offers benefits as different mechanisms of action might prevent the emergence of resistance. Moreover, it can be safely administered due to different toxicity profiles. To date, combined BTK/BCL-2 inhibitor treatment has been approved in the European Union, but not in the USA.
A phase II study evaluated 24 cycles of combined ibrutinib and venetoclax after 3 cycles of ibrutinib monotherapy. Both drugs were discontinued in patients who achieved MRD negativity at the end of this period, while those with MRD-positive disease received 12 additional combined cycles. At four years, all patients including high-risk individuals obtained uMRD as best response in 72 %, and the PFS rate was 94.5 % [10]. The PFS curves of patients with TP53-aberrant status and unmutated IGHV overlapped with those of patients without these high-risk features.
The largest combination study was the phase II CAPTIVATE trial that contained an MRD cohort (n = 164) and a fixed-duration cohort (n = 159). Each cohort received 12 cycles of ibrutinib and venetoclax. In the MRD cohort, this was followed by MRD-guided randomization. According to the most recent update, the fixed-duration cohort experienced a 4-year PFS rate of 79 % [11]. The phase III GLOW study led to the approval of ibrutinib plus venetoclax in many countries. Older patients and/or patients with comorbidities were randomized to either ibrutinib/venetoclax or chlorambucil/obinutuzumab. With respect to PFS, ibrutinib/venetoclax gave rise to a 78 % risk reduction (HR, 0.216; p < 0.001; Figure) [12].
Figure: GLOW study: progression-free survival with ibrutinib plus venetoclax vs. chlorambucil plus obinutuzumab
First-line triplets
Dr. Jain emphasized the time-limited nature of doublet and triplet therapies. This has some advantages such as decreased risk of resistance mutations and improved cost-effectiveness. Also, if patients progress after first-line combination regimens, unpublished data indicate that retreatment works.
In the first-line setting, several studies have investigated ibrutinib, acalabrutinib or zanubrutinib in addition to a backbone consisting of venetoclax and obinutuzumab (Ven + G). The CLL2-GIVe trial focused on ibrutinib plus Ven + G in patients with del(17p)/TP53-mutated CLL. In this population, the results were encouraging, with a 3-year PFS rate of 79.9 %, although the PFS curve suggested some late relapses [13]. Notable differences were observed regarding the type of aberration: while patients with TP53 mutation alone did not develop any relapses for more than three years, those with del(17p) with or without TP53 mutation fared considerably worse. Another study conducted in the high-risk setting tested the combination of acalabrutinib plus Ven + G. At cycle 16, high uMRD rates resulted across the total population and the patients with TP53 aberrations [14]. Similarly, the long-term follow-up of a phase II study has shown uMRD rates of 96 % and 92 % in the peripheral blood and bone marrow with zanubrutinib plus Ven + G [15].
As Dr. Jain pointed out, uMRD rates are numerically higher with second-generation BTK inhibitors than with ibrutinib, although this observation needs to be confirmed in a larger context. Ongoing phase III studies that are currently evaluating doublet and triplet regimens in the first-line setting will be reported over the next few years. Combination data for acalabrutinib plus venetoclax ± obinutuzumab and zanubrutinib plus venetoclax are awaited. Also, new BCL-2 inhibitors such as sonrotoclax and lisaftoclax are under development and will be assessed as part of combination strategies.
Source: Session “Optimizing initial therapy of CLL”, iwCLL 2023, 8th October 2023, Boston, USA
REFERENCES
- Barr BM et al., Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv 2022; 6(11): 3440-3450
- NCCN Guidelines Chronic Lymphocytic leukemia/Small Lymphocytic Lymphoma, version 3.2023
- Shanafelt TD et al., Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: updated results of the E1912 trial. Blood 2022; 140(2): 112-120
- Woyach J et al., Long-term results of Alliance A041202 show continued advantage of ibrutinib-based regimens compared with bendamustine plus rituximab (BR) chemoimmunotherapy. ASH 2021, abstract 639
- Al-Sawaf O et al., Minimal residual disease dynamics after venetoclax-obinutuzumab treatment: Extended off-treatment follow-up from the randomized CLL14 study. J Clin Oncol 2021; 39(36): 4049-4060
- Mato AR et al., A clinical practice comparison of patients with chronic lymphocytic leukemia with and without deletion 17p receiving first-line treatment with ibrutinib. Haematologica 2022; 107(11): 2630-2640
- Sharman JP et al., Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia 2022; 36(4): 1171-1175
- Byrd JC et al., Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: Results of the first randomized phase III trial. J Clin Oncol 2021; 39(31): 3441-3452
- Brown JR et al., Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 2022; 388(4): 319-332
- Jain N et al., Combined ibrutinib and venetoclax for first-line treatment of patients with chronic lymphocytic leukemia: 4-year follow-up data. ASH 2022, abstract 95
- Barr PM et al., Fixed-duration ibrutinib + venetoclax for first-line treatment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): 4-y follow-up from the FD cohort of the phase 2 CAPTIVATE study. J Clin Oncol 41, 2023 (suppl 16; abstr 7535)
- Kater AP et al., Fixed-duration ibrutinib-venetoclax in patients with chronic lymphocytic leukemia and comorbidities. NEJM Evid 2022; 1(7) DOI: 10.1056/EVIDoa2200006
- Huber H et al., Final analysis of the CLL2-GIVe trial: obinutuzumab, ibrutinib, and venetoclax for untreated CLL with del(17p)/TP53 mut. Blood 2023; 142(11): 961-972
- Ryan CE et al., Updated results from a multicenter, phase 2 study of acalabrutinib, venetoclax, obinutuzumab (AVO) in a population of previously untreated patients with CLL enriched for high-risk disease. Blood 2022; 140(Suppl 1): 837-838
- Soumerai JD et al., Long-term follow-up of multicenter phase II trial of zanubrutinib, obinutuzumab, and venetoclax (BOVen) in previously untreated patients with CLL/SLL. Hematol Oncol 2023; 41(S2): 233-235
© 2023 Springer-Verlag GmbH, Impressum
More posts
Issues in the management of CLL patients from an international point of view
The CLL Advocates Network (CLLAN) is a global network of patient advocacy organizations dedicated to improving the outcomes of patients with CLL through collaboration with national organizations. Principles guiding the work of CLLAN include the support of local communities, sharing of best practices and advocacy for better care and access.
CLL treatment in the real world: insights from across the globe
The analysis reported by Davids et al. at iwCLL 2023 examined the characteristics, treatment patterns and outcomes of a cohort of 1,102 real-world US patients with CLL receiving two or more lines of therapy. Data were obtained from the COTA real-world database. Second-line treatment was initiated between 2014 and 2021.
Overcoming resistance to targeted inhibitors
As covalent BTK inhibitors have been in use for the treatment of CLL in clinical practice for an extended period of time, different resistance mutations are being observed. Dr. Adrian Wiestner, MD, PhD, National Institutes of Health, Bethesda, USA, noted that mutations at progression are variable depending on the specific BTK inhibitor used, with the “classical” C481 mutations prevailing on ibrutinib and acalatinib treatment, while L528W mutations are mainly found in the context of zanubrutinib therapy.
Long-term results and other findings from clinical trials
Fixed-duration venetoclax plus rituximab (VenR; n = 194) was tested against bendamustine plus rituximab (BR) for six months (n = 195) in the global, open-label, randomized phase III MURANO study that enrolled patients with relapsed/refractory CLL. In the experimental arm, 6 cycles of rituximab were administered, and venetoclax monotherapy was taken for a total of 24 months.
Continuous BTK inhibition and combination regimens: present and future
Inhibitors of Bruton tyrosine kinase (BTK) have changed the standards of care in the setting of chronic lymphocytic leukemia (CLL). However, they do not eliminate leukemia, and undetectable residual disease is only very rarely achieved. Continuous treatment is therefore the standard approach, with the covalent BTK inhibitors ibrutinib, acalabrutinib and zanubrutinib being in widespread use as front-line single agents.
Preface – iwCLL 2023
The 20th International Workshop on chronic lymphocytic leukemia (iwCLL) was held in Boston, USA, and virtually from 6th–9th October 2023. This conference featured 11 sessions committed to discussing the management of patients with CLL world-wide and to creating progress regarding patient outcomes.