Management of WM patients previously exposed to BTK-inhibitors
Zanubrutinib in ibrutinib- or acalabrutinib-intolerant patients
Many patients with WM require continuous treatment with BTK inhibitors, but difficult-to-manage AEs often lead to treatment discontinuation [1]. Zanubrutinib is a potent and selective next-generation BTKi designed to minimize off-target kinase binding and associated side effects [2, 3]. Randomized phase III studies have shown that, in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) or WM, zanubrutinib has a more favorable safety profile than ibrutinib, particularly regarding cardiovascular toxicities [4, 5].
The BGB-3111-215 study (NCT04116437) is evaluating the tolerability and efficacy of zanubrutinib in patients with WM, CLL/SLL, MCL or marginal zone lymphoma (MZL), who did not tolerate prior BTKi-treatment (responded to BTKis but had adverse events). Ibrutinib- (Cohort 1) or acalabrutinib-intolerant patients (Cohort 2) are enrolled in this study. Eligible patients in both cohorts receive zanubrutinib (160 mg twice daily [BID] or 320 mg once a day [QD]) and are treated until disease progression or toxicity. Early results from the BGB-3111-215 study have already shown, that zanubrutinib was well tolerated in patients with B-cell malignancies who are intolerant to ibrutinib or acalabrutinib [3].
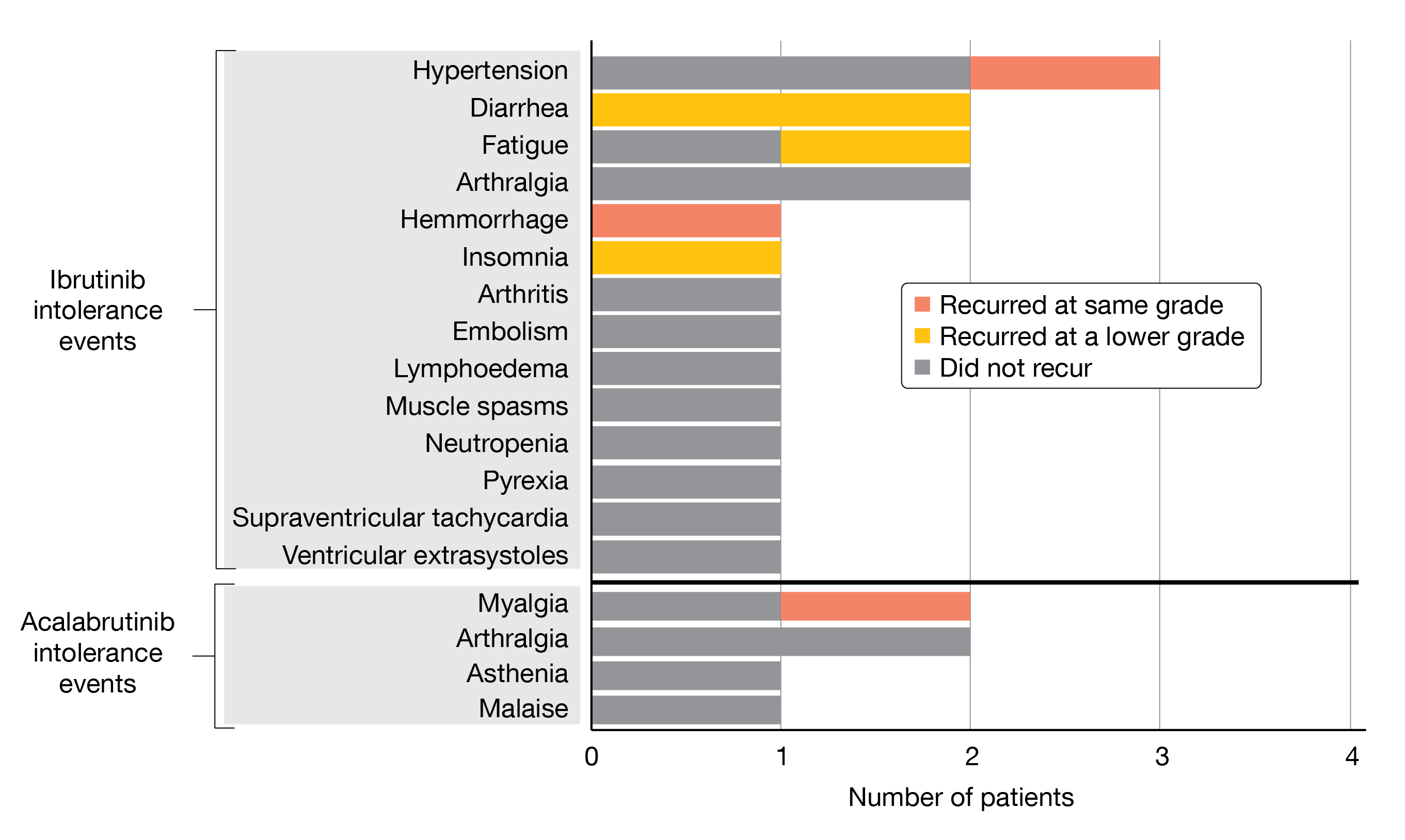
At iwWM 2022, Shadman et al. presented new data on patients with WM from this trial [6]. Eleven patients with WM were enrolled in the study, nine of them being intolerant to ibrutinib (Cohort 1), and two being intolerant to acalabrutinib (Cohort 2). Median age was 71 years and patients had received a median of two prior therapies (range, 1-12). The median duration of treatment was eleven months with ibrutinib and three months with acalabrutinib. At a median follow-up and median duration of treatment of 14,9 months (range, 6.5-20.5), 68 % of AEs that previously occurred from ibrutinib treatment and 83 % of AEs that resulted from acalabrutinib treatment did not recur with zanubrutinib. Events that recurred mostly occurred at lower grade in case of diarrhea, fatigue, and insomnia (Figure 1). In 45 % of patients, there was no recurrence of any prior BTKi-related intolerance AE on zanubrutinib. Cardiovascular AEs were less common in patients receiving zanubrutinib compared to ibrutinib. In terms of efficacy, data must be interpreted with caution since active disease was not a requirement for study enrollment. Nevertheless, WM patients treated with zanubrutinib either maintained (n = 1; 9.1 %) or improved (n = 10; 90.9 %) their disease status compared to baseline. However, one patient discontinued zanubrutinib because of myalgia, which recurred with the same severity as with prior acalabrutinib or ibrutinib therapy.
Consistent with a more selective BTK inhibition, zanubrutinib showed few side effects associated with off-target kinase activity in patients with WM, who were intolerant to ibrutinib and/or acalabrutinib. The authors concluded, that zanubrutinib represents a viable treatment option for patients with WM intolerant to other BTK inhibitors.
Figure 1: Recurrence and severity change of intolerance AEs in patients with WM under zanubrutinib-treatment.
Venetoclax as single agent in previously treated WM
B-cell lymphoma 2 (BLC2) is an essential regulator of apoptosis in both normal and malignant cells. It is overexpressed in WM cells, with similar levels of BCL2 expression regardless of CXCR4 mutation (CXCR4mut) [7]. Venetoclax is an oral BCL2 antagonist approved for the treatment of CLL and acute myeloid leukemia [8]. In a prospective phase I study, four patients with WM received venetoclax as a single agent, with all attaining a major response (MR) [9].
In a recently published phase II study (NCT02677324), patients with relapsed or refractory WM received venetoclax orally once daily at increasing doses: 200 mg for one week, 400 mg for another week, and 800 mg for up to two years [10, 11]. After each dose increase, the occurrence of tumor lysis syndrome was assessed, as previously described in CLL patients receiving venetoclax. In this study, a total of 32 WM patients were allocated to treatment, with 53 % of them presenting with CXCR4mut. The median age of WM diagnosis was 58 years and the age of ibrutinib initiation was 66 years. The overall response rate (ORR) was 84 %, with a lower ORR in patients with previous BTKi exposure (no prior BTKi: 93 %, prior BTKi: 75 %). Although the ORR was similar in CXCR4wt and CXCR4mut WM patients the VGPR was lower in CXCR4mut WM patients (29 % vs 12%, respectively). The median time to minor response was 1.9 months (95 % CI, 1.1-2.1), and the time to major response (TTMR) was 5.1 months (95 % CI, 4.7-8.2). Similarly, the time to minor and major responses were significantly longer in patients with prior BTKi exposure. Neither the ORR nor the time to response (TTR) were affected by a CXCR4mut. The median follow-up time was 30 months and the 12-month and 24-month PFS rates were 83% and 80%, respectively. Disease progression occurred in six patients within the first 24 months and in 13 patients after completion of venetoclax therapy, including ten between 24 and 36 months as well as three after 36 months. There were no statistically significant differences in PFS related to a prior BTKi exposure or CXCR4 mutational status.
Neutropenia was frequently observed and was effectively managed with G-CSF. One patient experienced a laboratory tumor lysis syndrome that was managed as an inpatient and responded to one dose of rasburicase and intravenous fluids. Seven patients (22 %) had venetoclax dose reduction, without affecting the ORR or PFS.
This study demonstrated that a venetoclax monotherapy resulted in a deep and durable response without unexpected AEs in patients with previously treated WM, including those previously treated with BTKis. An analysis of genomic material from the study is expected to be published next year.
BRUIN study: pirtobrutinib in pretreated WM
Until now, primary treatment options for WM include combination strategies with anti-CD20 monoclonal antibodies, chemotherapy, or proteasome inhibitors [12]. Additionally, covalent BTK inhibitors have emerged as standard therapy, but intolerance and acquired resistance, due to the BTK-C481 mutation in WM, are challenging [13, 14].
Pirtobrutinib is a highly potent, selective, non-covalent (reversible) BTKi [15]. Compared to ibrutinib, pirtobrutinib has demonstrated similar in vivo efficacy in wild-type BTK and superior efficacy in BTK-C481S in xenograft models [16]. Moreover, it was shown to be well tolerated with promising efficacy in poor-prognosis B-cell malignancy patients following prior therapy, including prior covalent BTK inhibitors [15]. The currently ongoing phase I/II BRUIN study (NCT03740529) evaluating pirtobrutinib consisted of a dose-escalation and expansion cohort (25 – 300 mg pirtobrutinib QD), and a cohort at the recommended phase II dose (RP2D) of 200 mg pirtobrutinib QD [15]. In addition to patients presenting with CLL/SLL, MCL, and other B-cell malignancies, 26 patients with WM were enrolled in this study. Co-primary endpoints were safety and tolerability, determination of the maximum tolerated dose and RP2D, pharmacokinetics, as well as efficacy of pirtobrutinib.
At iwWM 2022, M.L. Palomba presented the outcomes of the WM patient cohort [17]. The median number of prior therapies was three. Overall, 92 % of patients had a prior anti-CD20 antibody therapy, 89 % a prior chemotherapy, 69 % a covalent BTK inhibitor therapy, 4 % a PI3K inhibitor therapy and 69 % a prior BTKi therapy that was discontinued due to disease progression or toxicity. Efficacy assessment (n = 19) showed an ORR of 68 % (95 % CI, 44-87; 47% with a PR, 21 % with a minor response [MR)). In the BTK naïve patients (n = 6), 67 % (95 % CI, 22-96; PR, 67 %, MR, none) and in the prior BTKi treated patients (n = 13), 69 % (95 % CI, 39-91; PR, 39 %, MR, 31 %) achieved an ORR, respectively. If prior BTKi was discontinued due to disease progression, the ORR reached 63 % (95 % CI, 25-92; PR, 50 %, MR, 13 %); if prior BTKi was discontinued due to toxicity, the ORR reached 80 % (95 % CI, 28-100; PR, 20 %, MR, 60 %). Measurable tumor burden was reduced – 42 % of patients had a maximum percent change in IgM from baseline > 50%. At the time of data cut-off (September 27, 2020), ten of the 13 responding patients were still on treatment (Figure 2). With a median follow-up of 5.6 months, pirtobrutinib demonstrated a PFS of 71.7 % at seven months and 61.4 % at nine months.
The safety profile of pirtobrutinib was particularly notable in WM patients, as the only treatment-related adverse events (TEAEs) of grade 3 or higher was neutropenia (n = 2, 8 %). No TEAEs led to discontinuation of treatment.
The authors concluded that pirtobrutinib is highly active and well-tolerated drug in patients with previously treated WM, including those who have failed under prior covalent BTKi treatment.
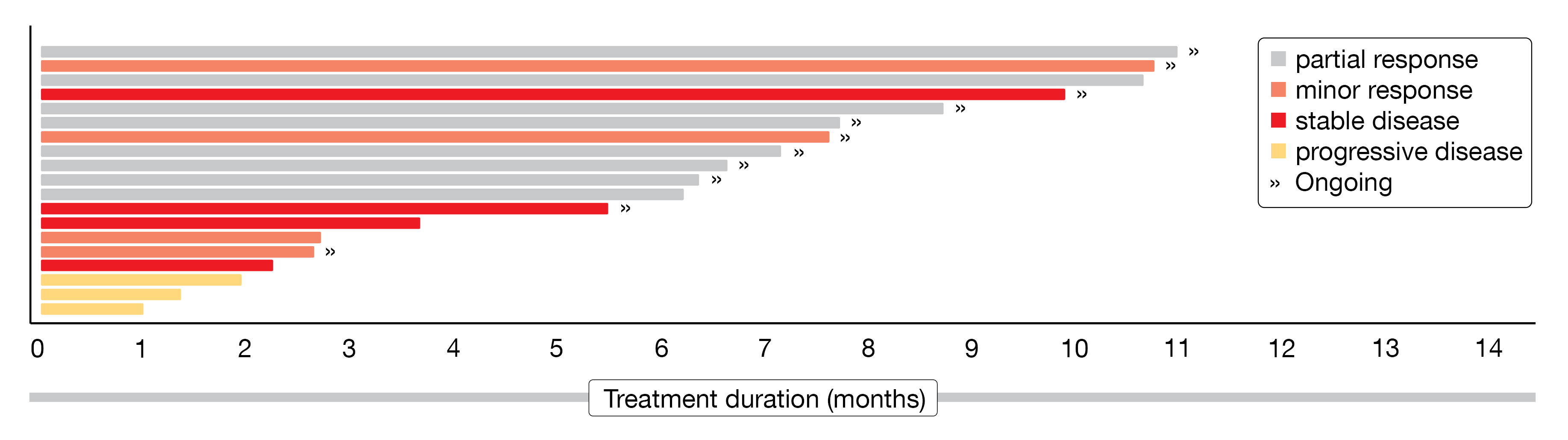
Figure 2: Swimmer plot on treatment duration of WM patients responding to pirtobrutinib.
Novel treatment approaches to WM
Ibrutinib plus venetoclax in treatment-naive WM
Both BTK inhibitors and BCL2 antagonists have been shown individually to be safe and effective treatments in WM [18]. The combination of the BTK inhibitor ibrutinib and the BCL2 antagonist venetoclax is safe and highly effective in CLL/MCL [19, 20]. In addition, preclinical work demonstrated a synergistic effect of ibrutinib and venetoclax in WM cell lines [21]. Based on these findings, a prospective single-arm, multicenter phase II study (NCT04273139) was designed to evaluate the combination of ibrutinib and venetoclax in therapy-naïve patients with WM. Results of this study were first presented at this year’s iwWM meeting by Jorge Castillo [22].
According to the study design, patients received 420 mg ibrutinib orally (PO) QD in Cycle 1. In Cycle 2, venetoclax was added to ibrutinib with a weekly dosage ramp-up, starting at100 mg in the first week, 200 mg in the second week, and 400 mg in the following two weeks. Ibrutinib (420 mg, PO, QD) and venetoclax (400 mg, PO, QD) were further administered in Cycles 3 to 24, with dose reductions allowed for toxicity. Tumor lysis syndrome was assessed after each stage of dose escalation. Patients were to discontinue study treatment only in the event of disease progression or unacceptable toxicity. In total, participants will be on the research study for up to two years on combined venetoclax and ibrutinib and four years of follow-up.
A total of 45 patients with WM were enrolled in the study, with a median age at treatment initiation of 67 years (range, 39-81). Participants were predominantly male (67 %) and 38 % (n = 17) of patients had a CXCR4 mutation (CXCR4mut). The study population presented a higher percentage of acquired von Willebrand disease in CXCR4mut (41 %) than in CXCR4wt (7 %), and lower ß2-microglobulin levels in CXCR4mut (2.8 mg/L) than in CXCR4wt (4.2 mg/L). Both median TTR and time to major response (TTMR) were 1.9 months, with a marginally significant difference (log-rank p = 0.048) in TTMR between CXCR4mut (2.8 months) and CXCR4wt (1.9 months) (Figure 3). The ORR reached 100 % (7 % MR, 53 % PR, 40 % VGPR) and the major response rate (MRR) was 93 % in the total population. However, the MRR was lower in patients with CXCR4mut versus CXCR4wt (88 % vs. 96 %). After a median follow-up of eleven months, the 12-month PFS rate was 92 % and the 12-month OS rate 95 %.
The safety profile showed predominantly grade 2 AEs. The most frequent grade ≥ 3 AE was neutropenia (n = 13). Moreover, three patients had grade ≥ 3 ventricular arrhythmia (grade 4, n = 1; grade 5, n = 2). Consequently, both therapies were discontinued, but patient follow-up will continue until the end of the five planned years.
In summary, the combination of ibrutinib and venetoclax resulted in a fast and deep response in previously untreated WM, with an even stronger response in patients with CXCR4mut . The high rate of ventricular arrhythmia prompted stopping study therapy, but follow-up off-therapy continues.
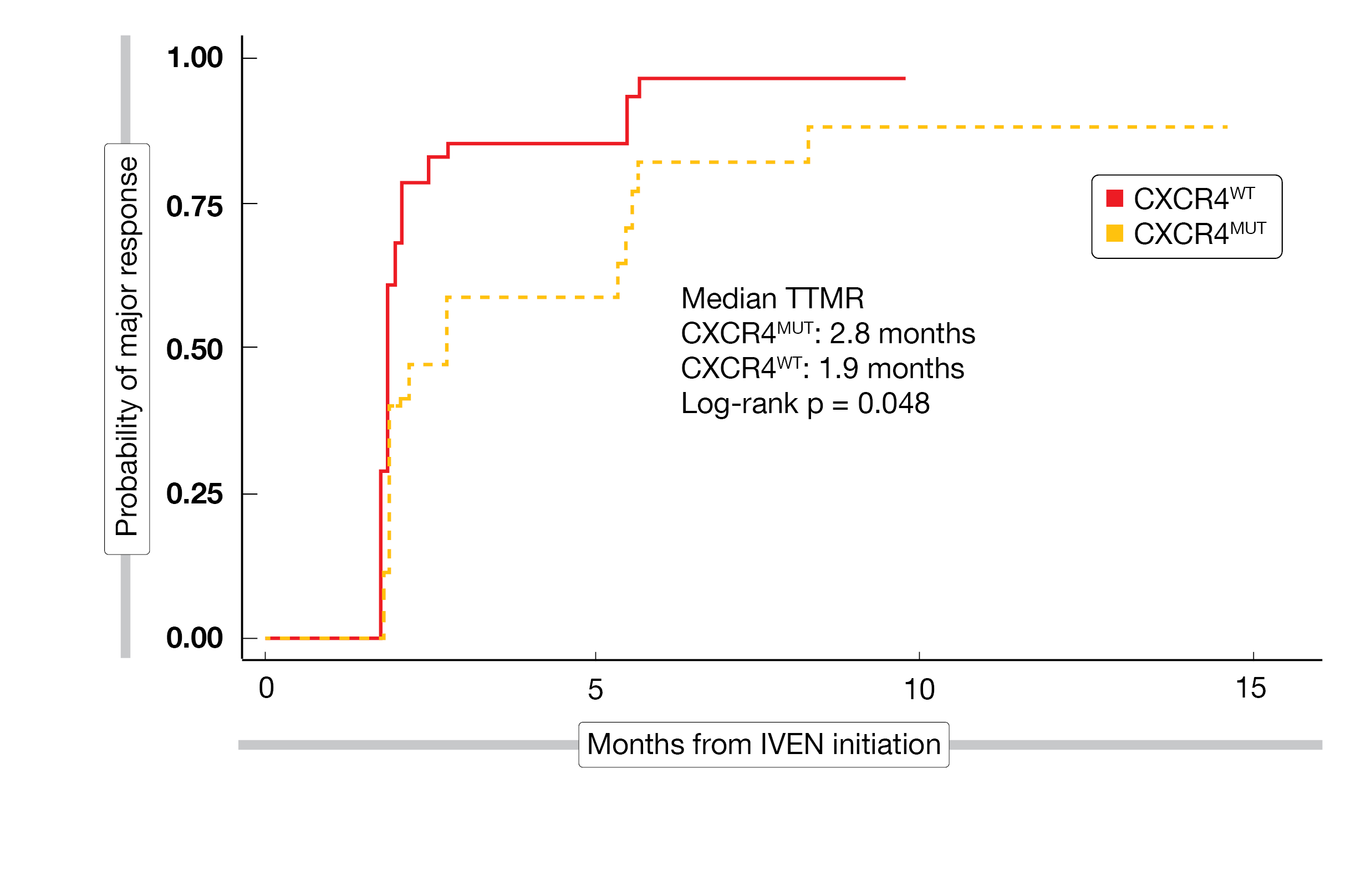
Figure 3: Time to major response in WM patients treated with ibrutinib plus venetoclax, assessed separately for patients with CXCR4wt or CXCR4mut.
Rituximab/acalabrutinib in WM with anti-MAG neuropathy
Monoclonal gammopathies encompass a spectrum of clonal plasma cell diseases that include monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma (MM), and Waldenström macroglobulinemia (WM). A known complication of monoclonal gammopathies is the peripheral neuropathy, which is challenging in terms of diagnosis and treatment [23]. Anti-myelin-associated glycoprotein (anti-MAG) antibodies are found in 40-50 % of patients with IgM monoclonal gammopathy [24]. Up to 50 % of patients with progressive neuropathy develop a significant disability within 10 to 15 years of diagnosis, severely impacting quality of life [25]. Current options for treatment of anti-MAG neuropathy include single agent rituximab or chemoimmunotherapy, but both result in poor and delayed responses and/or toxicity.
At iwWM 2022, S. Sarosiek presented the design of a phase II study (NCT05065554) evaluating the efficacy and safety of acalabrutinib plus rituximab for anti-MAG-mediated neuropathy in patients with WM or IgM mgUS [26]. It is expected that 33 patients will take part in this study, receiving rituximab (Days 1, 8, 15, 22) of Cycles 1 and 4 plus acalabrutinib (twice daily on Days 1-28) for 48 cycle or until disease progression or unacceptable toxicity. Participants will be followed for two years after completion of 48 cycles of treatment or until death. Main inclusion criteria include the presence of IgM monoclonal protein and anti-MAG antibodies, as well as sensory neuropathy with predominantly demyelinating features on nerve conduction tests (modified Rating Scale Score ≥ 1). Major exclusion criteria include serum IgM ≥ 4000 mg/dL, the fulfillment of other criteria for treatment besides neuropathy, no other known cause of peripheral neuropathy, a neuropathy for ≥ 5 years, or a prior exposure to other WM treatment except steroids, IVIg, or anti-CD20 antibodies > 90 days earlier.
Thus, the goal of this study is the assessment of the overall hematologic response rate (primary objective). The main secondary objective is to estimate the proportion of patients achieving improvement or stability on the I-RODS scale. Other secondary objectives are to evaluate various neuropathy rating systems/scales, including patient-directed rating systems and physician-directed rating systems.
Tirabrutinib in treatment-naive and previously treated WM
Tirabrutinib is a second-generation oral covalent BTKi with kinase selectivity comparable to or higher than other BTKis [27]. In patients with WM, a phase II study (ONO-4059-05 study) of tirabrutinib monotherapy at a daily dose of 480 mg led to its approval in Japan [28]. Updated results of this study after a median follow-up of two years were presented at iwWM 2022 by Koji Izutsu [29, 30].
The multicenter, open-label, single-arm study included patients with treatment-naive (Cohort A) or with relapsed/refractory (Cohort B) WM. Eligible patients were treated with tirabrutinib (480 mg, PO, once daily) until disease progression or unacceptable toxicity. The primary endpoint was IRC-assessed MRR (best response ≥ PR), while secondary endpoints included ORR, TTMR, PFS, OS, and safety.
A total of 27 patients (Cohort A, n = 18; Cohort B, n = 9) with a median age of 71 years and a median serum immunoglobulin M level of 3600 mg/dL were enrolled. Most patients (n = 22) showed a MYD88mut/CXCR4wt mutation status. At a median follow-up of two years, MRR was 94.4 % in treatment-naïve (Cohort A) and 88.9 % in the relapsed/refractory (Cohort B) WM patients, respectively. The median TTMR was 1.9 months/2.1 months in Cohorts A/B while the 2-year PFS reached 94.4 %/88.9 %, respectively. The 2-year OS rate was 100 % in both groups. The median PFS and median OS were not reached after a median follow-up of 23.8 months/25.4 months in Cohorts A/B, respectively.
The most common AEs of any grade in the total study population were rash (44.4 %), neutropenia (30 %) and nasopharyngitis (26 %). Eight bleeding events of grade 1 or 2 were reported (Cohort A, n = 5; Cohort B, n = 3). Atrial fibrillation occurred in two patients, one in each cohort, with one case being considered to be treatment related. All patients who showed either a complete response (n = 1) or a very good partial response (n = 8) were MYD88mut/CXCR4wt, but the impact of the mutational status remains unclear due to the small sample size.
In summary, this study demonstrated that tirabrutinib monotherapy is highly effective in both untreated and relapsed/refractory WM and has a manageable safety profile.
Orelabrutinib in relapsed/refractory WM patients
Orelabrutinib is a novel orally administered, potent, irreversible, and highly selective BTK-inhibitor. A nearly complete (99 %) BTK occupancy achieved with 150 mg orelabrutinib persisted for 24 hours, supporting once-daily dosing [31]. In December 2020, orelabrutinib received its first approval in China for the treatment of patients with mantle cell lymphoma or CLL/SLL, who have received at least one treatment in the past. Clinical development of orelabrutinib for various indications is underway in the USA and China [32].
At iwWM 2022 meeting, X. Cao presented the multicenter ICP-CL-00105 study (NCT04440059) of orelabrutinib in patients with relapsed or refractory WM who had at least one prior line of treatment [31, 33]. In this single-arm phase II study, orelabrutinib was administered orally at a daily dose of 150 mg until disease progression or unacceptable toxicity. The primary endpoint was the MRR, as assessed by the Independent Review Committee (IRC) according to IWWM-6 and NCCN guidelines; secondary endpoints included the MRR as assessed by investigator, ORR, duration of major response (DoMR), PFS, OS, and safety.
Among 66 relapsed or refractory WM patients assessed for eligibility, 47 eligible patients were evaluated for efficacy. The median age was 63 years, 83 % (n = 39) were MYD88mut/CXCR4wt and had a median of one prior line of therapy.
A decline in serum IgM levels (median: -80.4 %) from baseline as well as a durable improvement in hemoglobin levels in 87.2 % of patients (median maximal improvement at 33 g/L) were reported. The MRR attained 80.9 %, the ORR 89.4 % (21.3 % VGPR, 59.6 % PR, 8.5 % MR) and the DCR 97.9 %, respectively. The MRR was consistent in prespecified subgroups, but the presence of MYD88 mutation was associated with a high major response: 84.6 % in MYD88mut/CXCR4wt, 100 % in MYD88mut/CXCR4mut and 25.0 % in MYD88wt/CXCR4wt (Figure 4). At 12-months, a DoR of 88.8 %, a PFS rate of 85.1 %, an OS rate of 93.6 %, and a DoMR rate of 86.8 % were achieved.
Grade 3 treatment-related adverse events (TRAEs) occurred in 23.4 % of patients, one of them resulting in death because of a hepatitis B reactivation. The most common grade 3 or higher TEAEs were neutropenia (8.5 %), thrombocytopenia (6.4 %) and leukocytopenia (6.4 %).
Orelabrutinib has demonstrated a good efficacy and a manageable safety profile in patients with relapsed or refractory WM and has potential to become the preferred therapy for this patient population.
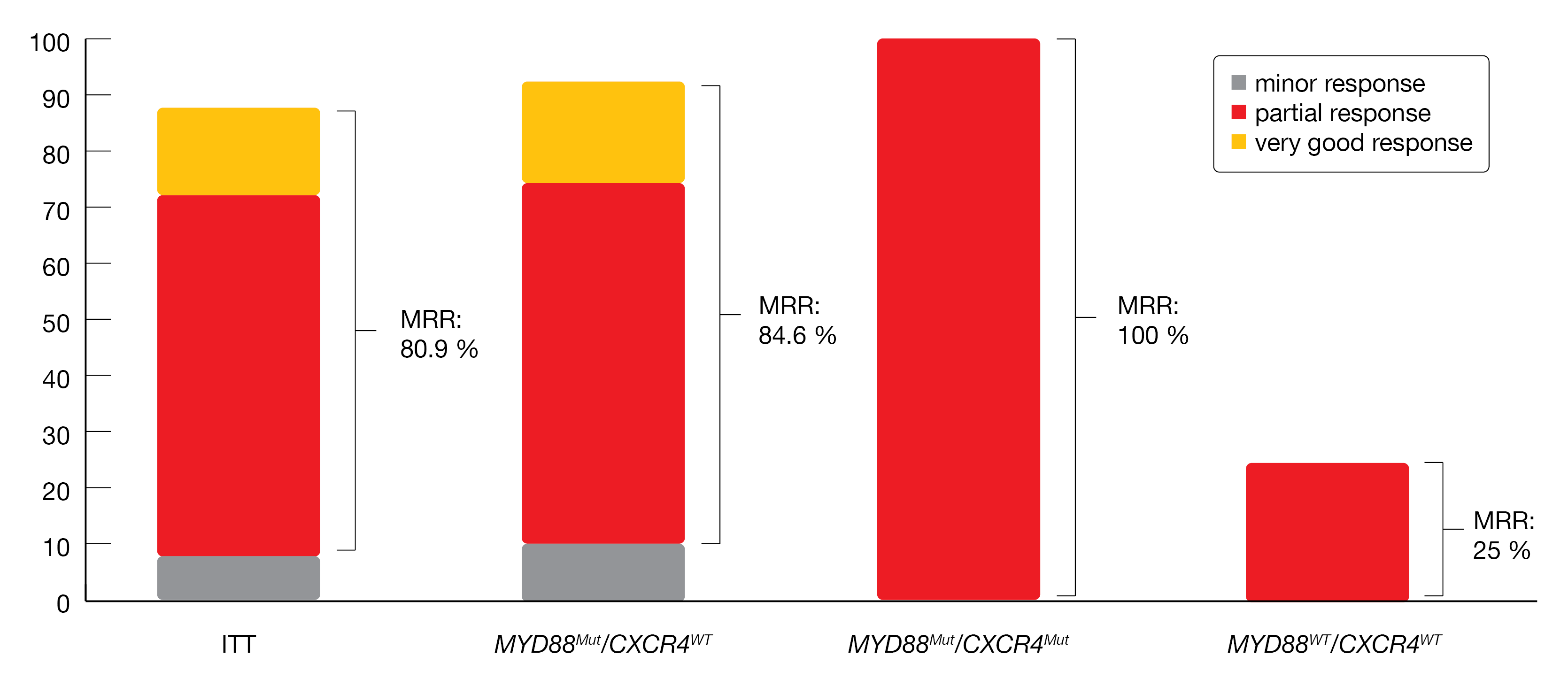
Figure 4: Response rates in different MYD88/CXCR4 genotype subgroups.
REFERENCES
- Ntanasis-Stathopoulos, I, et al., Current and novel BTK inhibitors in Waldenstrom’s macroglobulinemia. Ther Adv Hematol 2021; 12: 2040620721989586.
- Guo, Y, et al., Discovery of Zanubrutinib (BGB-3111), a Novel, Potent, and Selective Covalent Inhibitor of Bruton’s Tyrosine Kinase. J Med Chem 2019; 62(17): 7923-7940.
- Shadman, M, et al., Zanubrutinib in patients with previously treated B-cell malignancies intolerant of previous Bruton tyrosine kinase inhibitors in the USA: a phase 2, open-label, single-arm study. Lancet Haematol 2023: 10(1):e35-e45.
- Hillmen, P, et al., First interim analysis of alpine study: Results of a phase 3 randomized study of zanubrutinib vs ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. 2021: EHA 2021, Abstract lb1900.
- Tam, CSL, et al., ASPEN: Long-term follow-up results of a phase 3 randomized trial of zanubrutinib (ZANU) versus ibrutinib (IBR) in patients with Waldenström macroglobulinemia (WM). Journal of Clinical Oncology 2022; 40(16_suppl): 7521-7521.
- Shadman, M, et al., Zanabrutinib in patients intolerant to ibrutinib/acalabruinb. 2022; iwWM 2022, Session 12.
- Cao, Y, et al., The BCL2 antagonist ABT-199 triggers apoptosis, and augments ibrutinib and idelalisib mediated cytotoxicity in CXCR4 Wild-type and CXCR4 WHIM mutated Waldenstrom macroglobulinaemia cells. Br J Haematol 2015; 170(1): 134-138.
- Lasica, M, et al., Review of Venetoclax in CLL, AML and Multiple Myeloma. J Pers Med 2021; 11(6).
- Davids, MS, et al., Phase I First-in-Human Study of Venetoclax in Patients With Relapsed or Refractory Non-Hodgkin Lymphoma. J Clin Oncol 2017; 35(8): 826-833.
- Castillo, JJ, et al., Venetoclax in Previously Treated Waldenstrom Macroglobulinemia. J Clin Oncol 2022; 40(1): 63-71.
- Castillo, J, et al., Venetoclax in Previously Treated WM incl. BTK-inhibitor exposed patients 2022; iwWM 2022, Session 12.
- Brandhuber, B, et al., LOXO-305, a next generation reversible BTK inhibitor, for overcoming acquired resistance to irreversible BTK inhibitors. Clin Lymph Myel Leuk 2018(18): S216.
- Ababneh, O, et al., The Use of Bruton Tyrosine Kinase Inhibitors in Waldenstrom’s Macroglobulinemia. Clin Hematol Int 2022; 4(1-2): 21-29.
- Xu, L, et al., Acquired mutations associated with ibrutinib resistance in Waldenstrom macroglobulinemia. Blood 2017; 129(18): 2519-2525.
- Mato, AR, et al., Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet 2021; 397(10277): 892-901.
- Munir, T, et al., Pirtobrutinib, A Highly Selective, Non-Covalent (Reversible) BTK Inhibitor In Previously Treated CLL/SLL: Updated Results From The Phase 1/2 BRUIN Study. 2022: British Society for Haematology; Manchester, UK; 3-5 April 2022, BSH22-PO55.
- Palomba, ML, et al., Pirtobrutinib in previously treated WM patients with covalent BTK-inhibitors. 2022; iwWM 2022, Session 12.
- Castillo, JJ, et al., Management of Waldenstrom macroglobulinemia in 2020. Hematology Am Soc Hematol Educ Program 2020; 2020(1): 372-379.
- Jain, N, et al., Ibrutinib and Venetoclax for First-Line Treatment of CLL. N Engl J Med 2019; 380(22): 2095-2103.
- Tam, CS, et al., Ibrutinib plus Venetoclax for the Treatment of Mantle-Cell Lymphoma. N Engl J Med 2018; 378(13): 1211-1223.
- Paulus, A, et al., Waldenstrom macroglobulinemia cells devoid of BTK(C481S) or CXCR4(WHIM-like) mutations acquire resistance to ibrutinib through upregulation of Bcl-2 and AKT resulting in vulnerability towards venetoclax or MK2206 treatment. Blood Cancer J 2017; 7(5): e565.
- Castillo, J, et al., Venetoclax in combination with ibrutinib for WM. 2022; iwWM 2022, Session 14.
- Chaudhry, HM, et al., Monoclonal Gammopathy-Associated Peripheral Neuropathy: Diagnosis and Management. Mayo Clin Proc 2017; 92(5): 838-850.
- Stork, AC, et al., Prevalence, specificity and functionality of anti-ganglioside antibodies in neuropathy associated with IgM monoclonal gammopathy. J Neuroimmunol 2014; 268(1-2): 89-94.
- Campagnolo, M, et al., Limitations in daily activities and general perception of quality of life: Long term follow-up in patients with anti-myelin-glycoprotein antibody polyneuropathy. J Peripher Nerv Syst 2019; 24(3): 276-282.
- Sarosiek, S, et al., Acalabrutinib and Rituximab for WM and IGM RD PN. 2022; iwWM 2022, Session 14
- Kaptein, A, et al., Potency and Selectivity of BTK Inhibitors in Clinical Development for B-Cell Malignancies. Blood 2018; 132 1871.
- Sekiguchi, N, et al., A multicenter, open-label, phase II study of tirabrutinib (ONO/GS-4059) in patients with Waldenstrom’s macroglobulinemia. Cancer Sci 2020; 111(9): 3327-3337.
- Izutsu, K, et al., Tirabrutinib in treatment-naive and previously treated WM. 2022; iwWM 2022, Session 15.
- Sekiguchi, N, et al., Two-year outcomes of tirabrutinib monotherapy in Waldenstrom’s macroglobulinemia. Cancer Sci 2022; 113(6): 2085-2096.
- Cao, XX, et al., Evaluation of orelabrutinib monotherapy in patients with relapsed or refractory Waldenstrom’s macroglobulinemia in a single-arm, multicenter, open-label, phase 2 study. EClinicalMedicine 2022; 52: 101682.
- Dhillon, S, Orelabrutinib: First Approval. Drugs 2021; 81(4): 503-507.
- Cao, XX, et al., Phase II study of Orelabrutinib in relapsed/refractory WM. 2022; iwWM 2022, Session 15
© 2023 Springer-Verlag GmbH, Impressum
More posts
Emergent BTKi treatments in WM
Additionally to ibrutinib, to date the only once-daily BTK inhibitor approved in the USA and the European Union either as monotherapy or in combination with RTX for patients with WM [1], other BTKis, such as acalabrutinib and zanubrutinib, are now emerging as potential therapeutic alternatives. Acalabrutinib is an emergent, potent, and selective BTKi, which has received accelerated approval by the US FDA for the treatment of adult patients with relapsed or refractory (R/R) MCL and is in clinical development for CLL and DLBCL.
BTK inhibition in Waldenström’s macroglobulinemia: trial updates and biomarker analysis
The open-label, multicenter, randomized phase III ASPEN trial was set up to assess the efficacy and safety of the potent, selective, irreversible next-generation BTK inhibitor zanubrutinib in Waldenström’s macroglobulinemia (WM). Cohort 1 of the study included patients with MYD88-mutated disease (n = 201); here, zanubrutinib was compared to ibrutinib after 1:1 randomization.
Management of WM patients previously exposed to BTK-inhibitors
Many patients with WM require continuous treatment with BTK inhibitors, but difficult-to-manage AEs often lead to treatment discontinuation. Zanubrutinib is a potent and selective next-generation BTKi designed to minimize off-target kinase binding and associated side effects.
New insights into BTKi treatment of Waldenström‘s macroglobulinemia
Waldenström’s macroglobulinemia (WM) is a low-grade non-Hodgkin B-cell lymphoplasmacytic lymphoma, characterized by the accumulation of clonal lymphoplasmacytic cells secreting monoclonal IgM protein in the bone marrow and other organs. WM is a lymphoma accounting for only 1–2 % of all hematologic tumors, with an annual incidence of three to four cases per million people in the USA and Europe, classifying it as a rare disease.
Preface – iwWM 2022
The 11th International Workshop on Waldenström’s Macroglobulinemia (iwWM), held in Madrid, Spain, and virtually from 27th–30th October 2022, featured 20 sessions with more than 100 presentations.