Recent progress in the treatment of neuroendocrine tumors
Positive efficacy with 177Lu-octreotate in PanNET: Phase II OCLURANDOM
Five systemic therapeutic options are currently approved for advanced pancreatic neuroendocrine tumors (PanNET): Streptozotocine-based chemotherapy, everolimus, sunitinib, lanreotide, and PRRT with 177Lu-DOTA-octreotate (OCLU). For PRRT, data from retrospective studies have reported partial responses in advanced PanNET patients [1]. So far, no prospective randomized trial has reported data regarding PRRT in patients with advanced PanNET. OCLURANDOM, a phase II study, is the first randomized phase II trial evaluating PRRT with OCLU in advanced, sporadic PanNET patients with an incidence of less than 1 in 100,000 (NCT02230176) [2].
Advanced PanNET patients who had progressed in the last 12 months and were positive on somatostatin receptor scintigraphy were randomized 1:1 to either 4 cycles of OCLU (7.4 GBq/cycle at 8±1 week intervals) or sunitinib (SUN). The primary endpoint was the PFS rate at 12 months; key secondary endpoints included median PFS and safety.
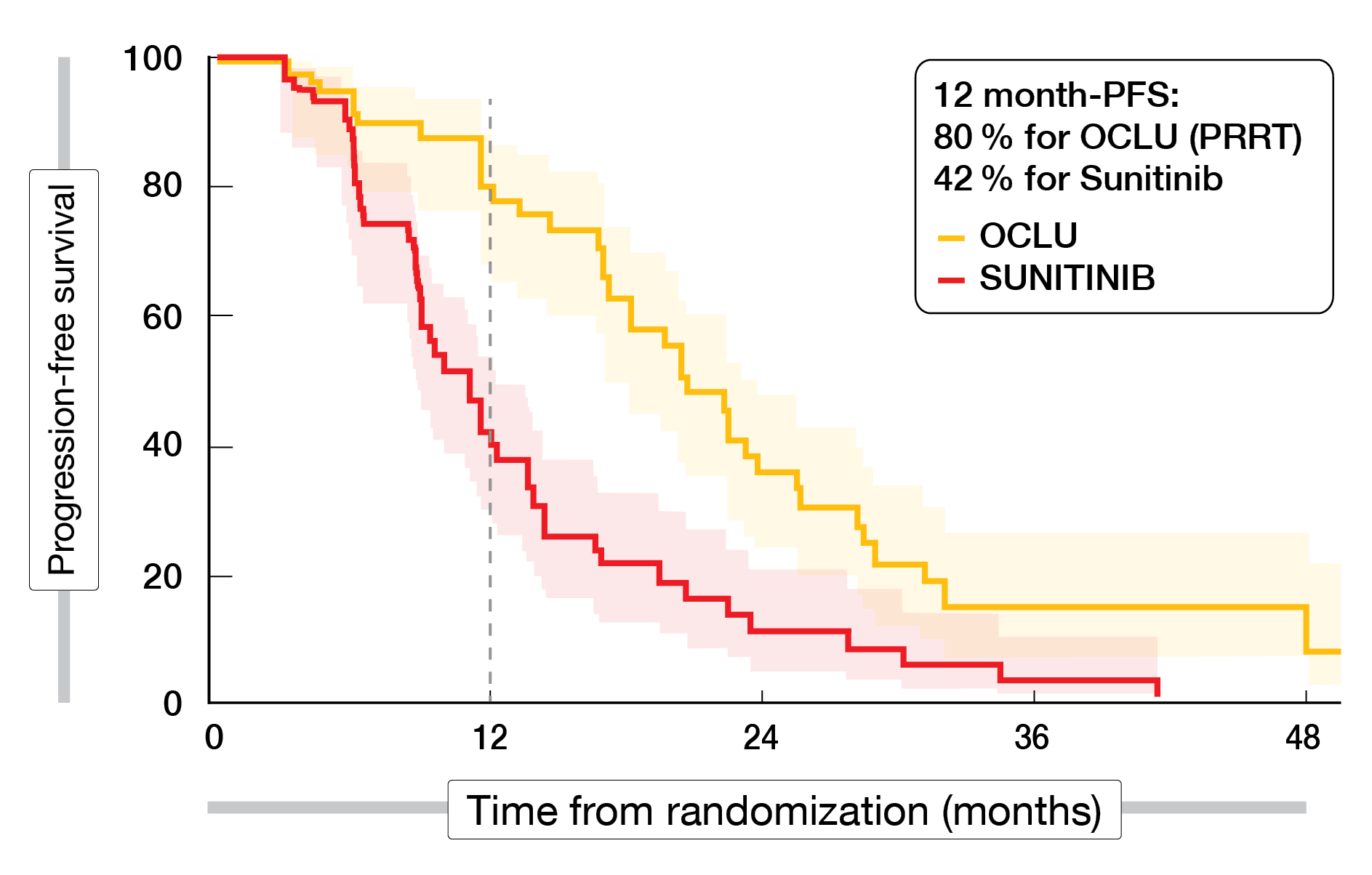
In 5 years, 84 patients were enrolled in the study (41 OCLU; 43 SUN) with the baseline characteristics well balanced between the study arms. Almost 90 % of patients received 4 cycles of OCLU. After a median follow-up of 40 months, the primary endpoint was met, with a 12-month-PFS rate of 80.5 % in the OCLU arm vs. 42 % in the SUN arm (Figure 1).
The median PFS was 20.7 months in the OCLU arm vs. 11 months in the SUN arm. Among the total patients treated, grade 3/4 AEs were experienced in 44 % of patients in the OCLU arm vs. 63 % in the SUN arm, with the most frequent AEs being blood-related, digestive, and hypertension. The OCLU arm had one case each of thymoma, basal cell carcinoma, and myelodysplastic syndromes (MDS) compared to the SUN arm, which had one case each of gastrointestinal stromal tumor (GIST) and MDS.
In conclusion, 177Lu- DOTA-Octreotate showed positive promising efficacy and a tolerable safety profile compared to sunitinib in the first PRRT randomized trial in advanced progressive SSR-positive PanNET patients. These positive PRRT data can potentially change the treatment paradigm in progressive advanced SSR+ PanNET patients by starting PRRT treatment prior to sunitinib. Ongoing randomized phase 3 trials, COMPETE (NCT03049189) & COMPOSE (NCT04919226), assessing PRRT with 177Lu-Edotreotide in advanced PanNETs as part of a larger GEP-NETs patient population will help confirm the efficacy and safety of 177Lu-based PRRT and provide the best treatment sequencing in this patient population.
Figure 1: Progression-free survival curves of patients treated with either 177Lu-DOTA-octreotate (OCLU) or sunitinib (SUN) in the OCLURANDOM trial
225Ac-DOTATOC shows promise in upfront and salvage PanNETs treatment
Studies have demonstrated that alpha-PRRT (PRART) elicits tumor responses in patients who have become resistant or are unsuitable to beta-PRRT [3-5]. PRART using 225Ac-labeled SSA has shown promising results and improved OS, even in patients refractory to prior 177Lu-PRRT treatment, with transient and acceptable adverse effects [6]. Kulkarni et al. presented data from a retrospective analysis that estimated the response at 3 months and PFS after 2-4 cycles of PRART using 225Ac-DOTATOC in progressive metastatic neuroendocrine neoplasms (NENs) [7].
A total of 41 patients with confirmed NENs on 68Ga-DOTATOC PET/CT were treated with PRART using 2-4 cycles of 225Ac-DOTATOC at 8-12-week intervals. Of these patients, 36 had progressed after previous PRRT (2-11 cycles) using beta-emitters (Lu-177 / Y-90) and received PRART as a salvage treatment. On the other hand, 5 patients with disseminated liver metastases received upfront PRART. The primary endpoint was objective response 3 months post-therapy measured by RECIST 1.1. Secondary endpoints included OS, PFS, and toxicity after upfront or salvage PRART.
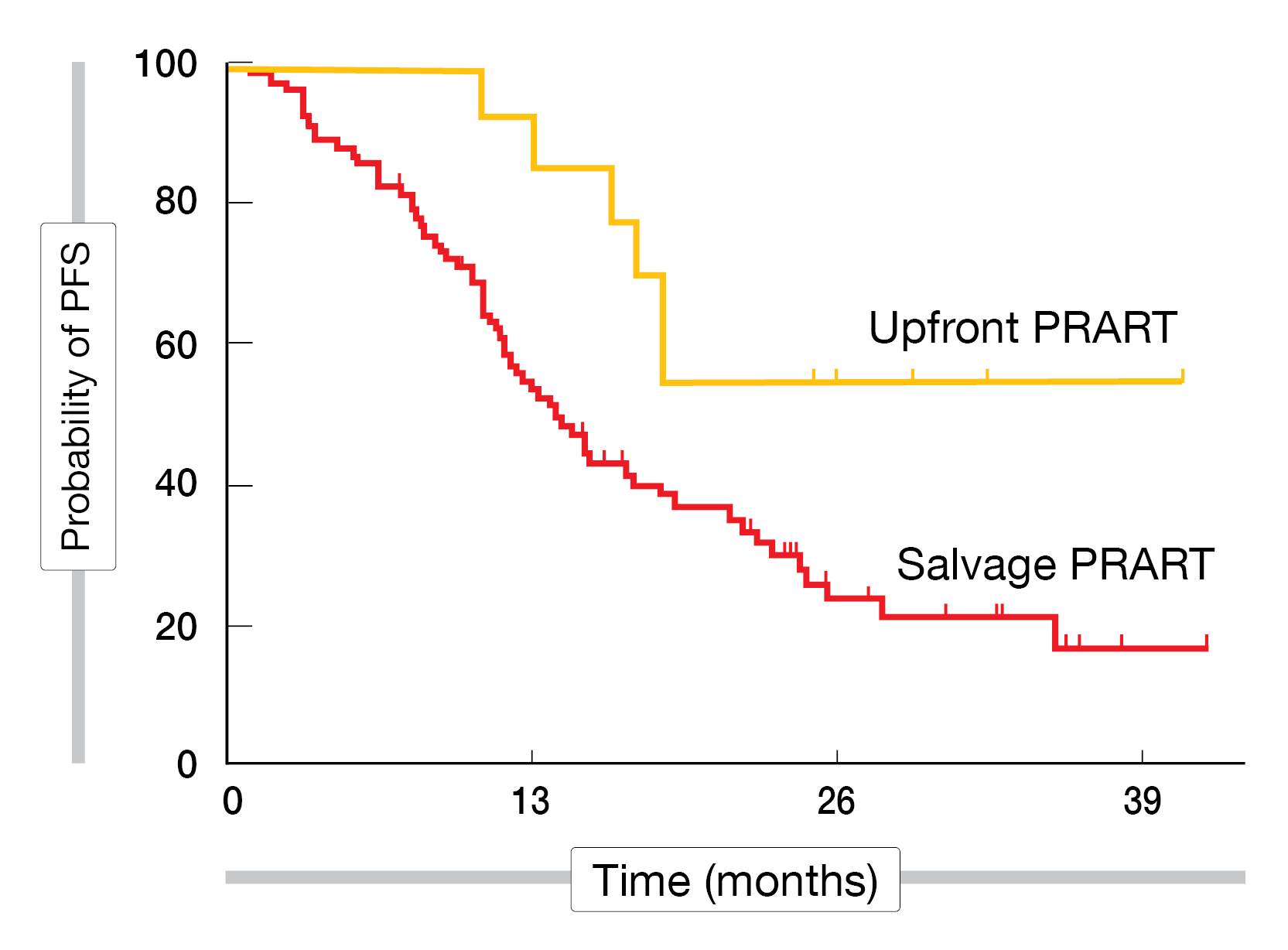
All patients receiving upfront PRART and 50 % receiving salvage PRART demonstrated a PR of the disease at 3 months post-therapy. SD was seen in 38.9 %, and PD in 11.1 % after salvage PRART. All patients tolerated PRART well, with grade 1-2 anemia in 34 %, bicytopenia in 27 %, and pancytopenia in 7 % of all patients treated. Two patients experienced mild renal dysfunction (grade 2). There was no grade 3-4 hematological or renal toxicity or worsening of hepatic function post-therapy. The median PFS after salvage PRART was 14 months (range 9-19). The median PFS after upfront PRART and the median OS in all patients had not been reached (Figure 2).
In conclusion, PRRT using 225Ac-labeled DOTATOC was shown to be efficacious with a relatively good safety profile in the upfront as well as salvage treatment settings in patients with NENs. However, prospective clinical studies are required to optimally select patients for the PRART upfront therapy or in the beta-emitter refractory setting
Figure 2: Progression-free survival curves of patients treated with PRART either as upfront or salvage treatment
177Lu-DOTATATE shows similar results in pancreatic and midgut NETs: Results of the SEPTRALU study
The phase 3 NETTER-1 trial established the efficacy and safety of 177Lu-DOTATATE therapy in patients with advanced midgut grade 1-2, SSR-positive NETs progressing to long-acting octreotide [8]. However, no phase 3 trial data exist for patients with PanNET. SEPTRALU is a national, multi-center registry of 533 patients with advanced NET (PanNET: n=187) treated with 177Lu-DOTATATE in clinical practice to analyze the efficacy and safety of 177Lu-DOTATATE [9]. Previous treatments, number of treatment cycles per patient, response, survival, and toxicity were analyzed.
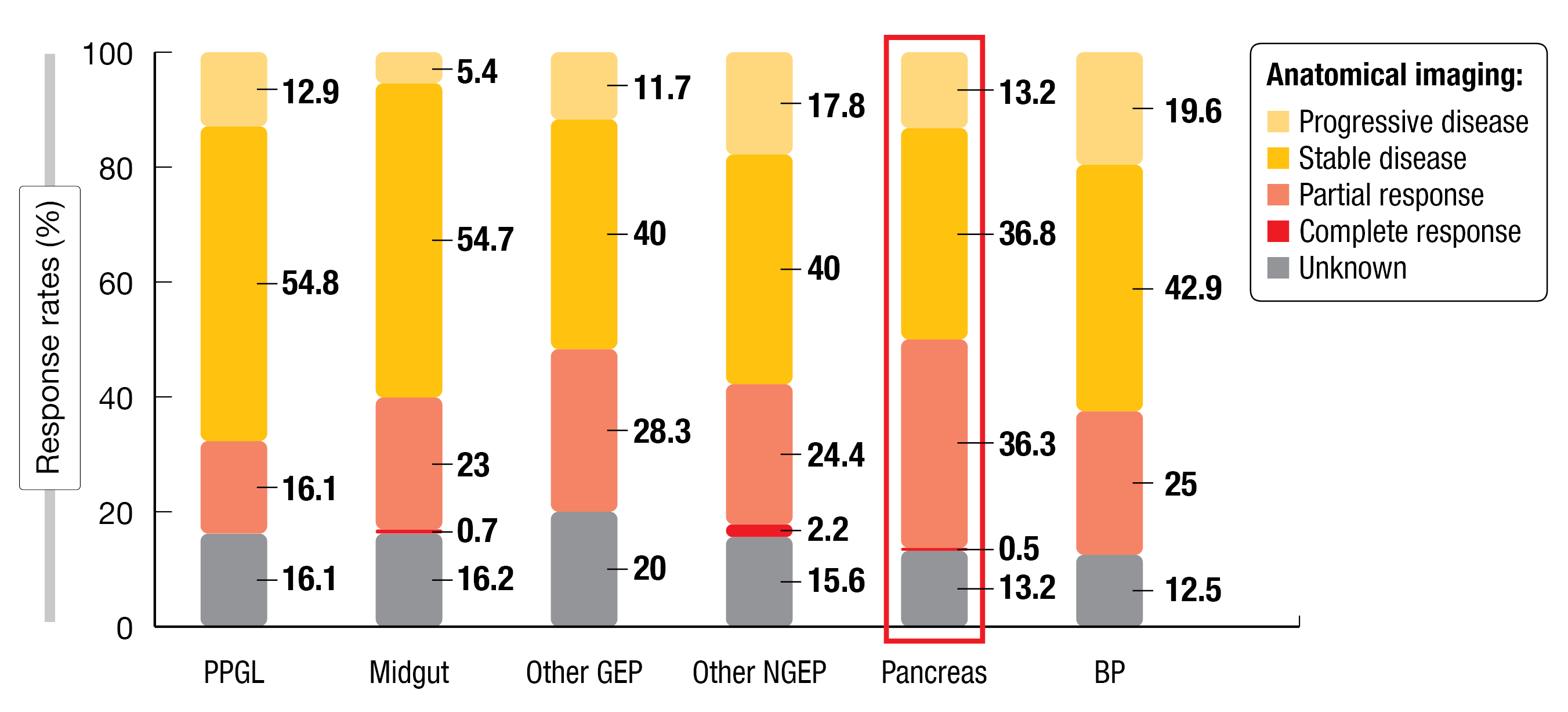
Among the 187 PanNET patients analyzed, 177Lu-DOTATATE was usually performed as the third (31 %) or later (46 %) treatment option, with the previous treatments being SSAs (92 %), surgery (48 %), everolimus (43 %) or tyrosine kinase inhibitors (36 %). The overall response rate (ORR; complete and partial response) was 36.8 %, and the disease control rate (DCR; ORR + stable disease) was 73.6 %. Progression occurred in 13.2 % of patients. Conversely, midgut NET patients had an ORR of 23.7 % and a DCR of 78.4 % (Figure 3).
The median PFS was 28.9 months, and the median OS was 41 months. The median PFS results were worse for patients who underwent more lines of treatment (0-1 lines: 66.2 months; 2 lines: 17 months; >2 lines: 14.6 months). The better response for PanNET patients did not translate into a better PFS or OS compared to NETs of other locations. Grade 1-2 toxicities included nausea (36 %), hematological (31 %), and emesis (26 %); grade 3-4 toxicities were hematological (5 %), emesis (1 %), and nephrotoxicity (1 %).
In conclusion, advanced PanNET patients treated with 177Lu-DOTATATE in this clinical practice series showed similar efficacy and safety data to those reported in midgut NET patients.
Figure 3: Response rates among NET patients after 177Lu-DOTATATE treatment based on histology type (PPGL, pheochromocytomas and paragangliomas; BP, Bronchopulmonary)
REFERENCES
- Wheless M, Das S. Systemic Therapy for Pancreatic Neuroendocrine Tumors [published online ahead of print, 2022 Aug 27]. Clin Colorectal Cancer. 2022;S1533-0028(22)00088-3.
- Taieb D et al. Results from OCLURANDOM: the first multicentric randomized phase II trial investigating the antitumor efficacy of peptide receptor radionuclide therapy with lutetium-177 octreotate (OCLU) in patients with unresectable progressive neuroendocrine pancreatic tumors. EANM 2022 (Oral Abstract OP-154)
- Kratochwil, C et al. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur J Nucl Med Mol Imaging 2014:41:2106–2119.
- Zhang J et al. Peptide receptor radionuclide therapy using 225Ac-DOTATOC achieves partial remission in a patient with progressive neuroendocrine liver metastases after repeated β-emitter peptide receptor radionuclide therapy. Clin. Nucl. Med 2020;45:241-243.
- Ballal S et al. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: first clinical experience on the efficacy and safety. Eur J Nucl Med Mol Imaging 2020;47:934–946.
- Ballal S et al. Survival Outcomes in Metastatic Gastroenteropancreatic Neuroendocrine Tumor Patients receiving Concomitant 225Ac-DOTATATE Targeted Alpha Therapy and Capecitabine: A Real-world Scenario Management Based Long-term Outcome Study. J Nucl Med. 2022;jnumed.122.264043
- Kulkarni H et al. Peptide Receptor Alpha-particle-emitting Radionuclide Therapy of Progressive Metastatic Neuroendocrine Neoplasms Prolongs Progression-free Survival in an Upfront as well as Salvage Treatment Setting. EANM 2022 (Oral Abstract OP-754)
- Strosberg J et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med 2017; 376:125-135
- Mitjavila-Casanovas M et al. 177Lu-DOTATATE therapy in advanced pancreatic neuroendocrine tumors: data from 187 patients of the national registry SEPTRALU. EANM 2022 (Oral Abstract OP-755)
© 2023 Springer-Verlag GmbH, Impressum
More posts
Neuroendocrine tumor imaging updates
Guideline recommendations for peptide receptor radionuclide therapy (PRRT) using 177Lu- DOTA-0-Tyr3-Octreotate (DOTATATE) in patients with neuroendocrine tumors (NETs) include 3-5 cycles with a dose ranging from 5.5-7.4 GBq per cycle with 6-12 weeks intervals [1]. While PET with radionuclide-labeled somatostatin analogs (SSAs) is mandatory before PRRT, interim PET imaging is not routinely recommended.
Latest developments in prostate cancer treatment
The positive efficacy and safety data of 177Lu-PSMA-617 in the treatment of mCRPC patients from the Lu-PSMA and VISION trials led to its FDA approval and designation as a breakthrough therapy for later lines of mCRPC treatment [1,2]. 223Ra-dichloride (223RaCl2) is a targeted α-therapy and prolongs OS in patients with bone-predominant mCRPC [3].
Advances in PSMA radiotracers for prostate cancer imaging
Prostate-specific membrane antigen (PSMA)-targeting positron emission tomography/computed tomography (PET/CT) imaging is increasingly used to characterize prostate cancer (PCa). However, in Europe, there is still an unmet need for radiotracers to localize biochemical recurrences in PCa. The phase III PYTHON trial is designed to establish the efficacy and safety of [18F]DCFPyL- compared to [18F]Flurocholine-PET/CT in patients with first biochemical recurrence after initial definitive therapy (prostatectomy, external beam radiotherapy or brachytherapy) for histopathologically confirmed prostate adenocarcinoma per original diagnosis [1].
Preface – EANM 2022
Dear Colleagues, After two years of virtual events, the nuclear medicine and oncology communities were excited to meet each other in person at the 35th Annual Congress of the European Association of Nuclear Medicine, held in Barcelona, Spain, and virtually from 15th – 19th October 2022. The event has celebrated its status as the world’s leading meeting for nuclear medicine, with more than 7000 participants from 121 countries presenting and discussing groundbreaking clinical updates and scientific research advancements in several disease areas.