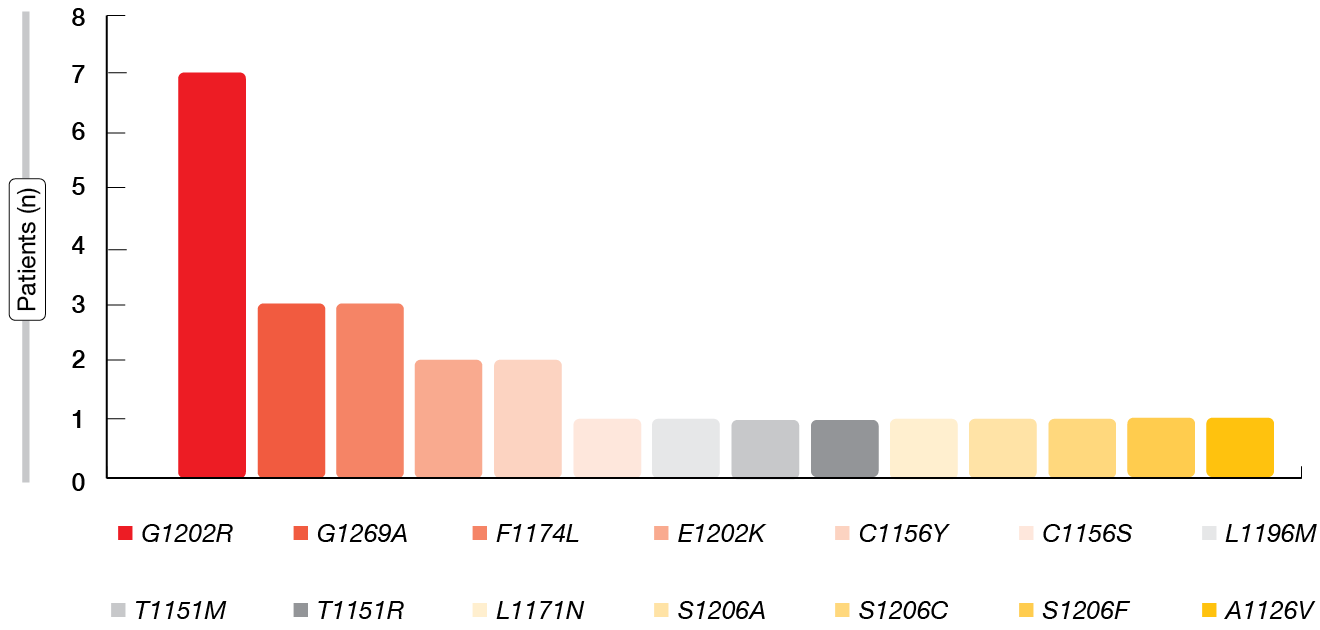KRAS, HER2 & ALK: targeted options and sequencing issues
Deep responses with sotorasib: CodeBreak 100
The KRAS p.G12C mutation is a key oncogenic driver occurring in approximately 13 % of lung adenocarcinomas [1] and is associated with poor patient outcomes. The first-in-class, highly selective and irreversible KRASG12C inhibitor sotorasib has shown durable clinical benefit in a cohort of 59 heavily pretreated patients with NSCLC included in phase I of the CodeBreaK 100 study [2]. Li et al. presented the results from the NSCLC cohort of the registrational, open-label, single-arm, phase II CodeBreaK 100 trial at the WCLC 2020 Congress [3]. In this part of the study, 126 patients from 11 countries with KRAS p.G12C-mutated, locally advanced or metastatic NSCLC who had progressed on prior standard therapies were treated with sotorasib 960 mg daily orally until disease progression. Almost all of them were current or former smokers. One prior line of systemic anticancer therapy had been administered in 42.9 %, while 34.9 % of patients had received two lines and 22.2 % three lines. Notably, 81 % had progressed on platinum-based chemotherapy and PD-(L)1 inhibitor treatment. The objective response rate (ORR) constituted the primary endpoint.
After a median follow-up of 12.2 months, confirmed ORR was 37.1 %, with 2.4 % and 34.7 % of patients achieving complete and partial responses, respectively. The disease control rate was 80.6 %. Tumor shrinkage of any magnitude occurred in 81 %; among responders, the median percentage of best tumor shrinkage amounted to 60 %. Moreover, the trial demonstrated early and durable responses to sotorasib. Median time to objective response and median duration of response were 1.4 and 10.0 months, respectively. Forty-three percent of responders remained on treatment without progression as of the data cutoff. Median progression-free survival was estimated at 6.8 months.
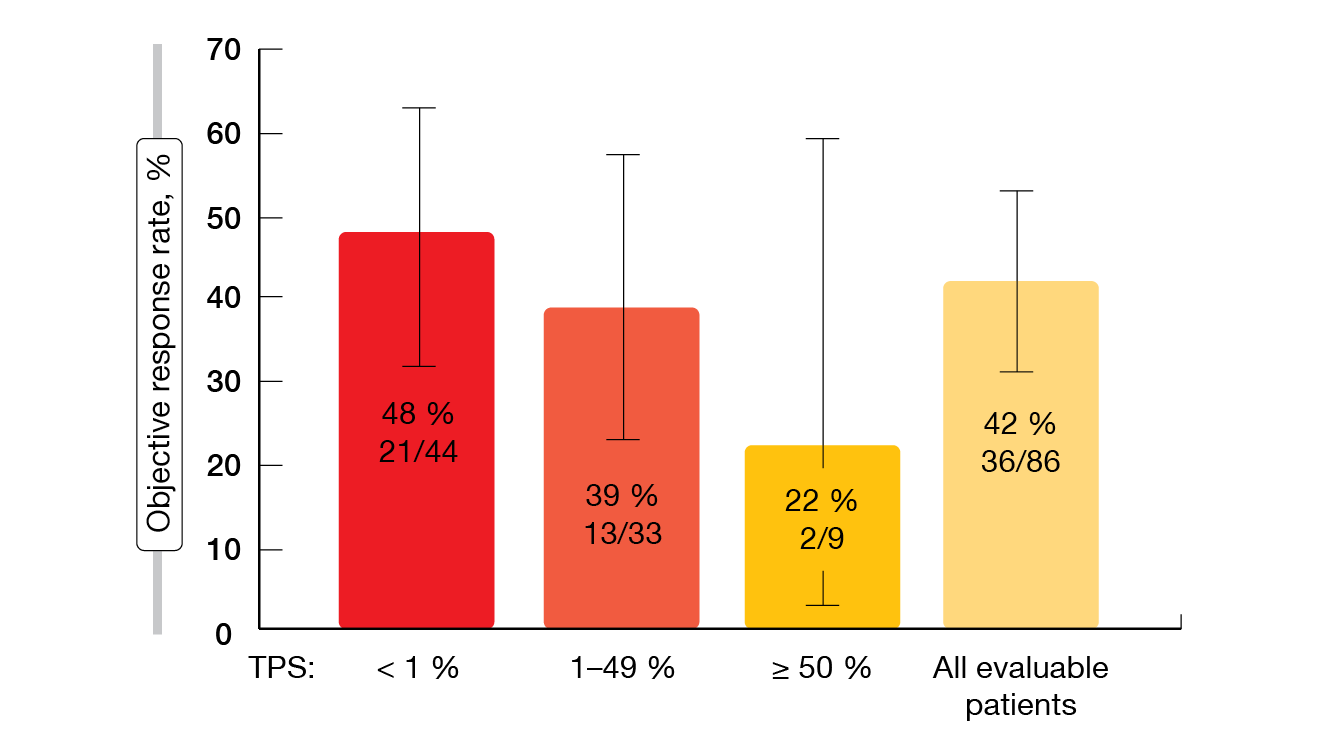
Treatment-related adverse events were generally mild and manageable, with low rates of grade 3 and 4 events (19.8 % and 0.8 %, respectively). Treatment discontinuation and dose modifications became necessary in 7.1 % and 22.2 %, respectively. The most commonly reported AEs comprised diarrhea, nausea as well as increases in ALT and AST levels. According to the exploratory biomarker analyses included in CodeBreaK 100, responses to sotorasib occurred in patients with low or negative PD-L1 expression (Figure 1) and independently of the STK11/KEAP1 mutation status. The confirmatory phase III CodeBreaK 200 trial comparing sotorasib with second-line docetaxel is currently enrolling (NCT04303780).
Figure 1: Responses to sotorasib therapy according to PD-L1 expression levels
DESTINY-Lung01
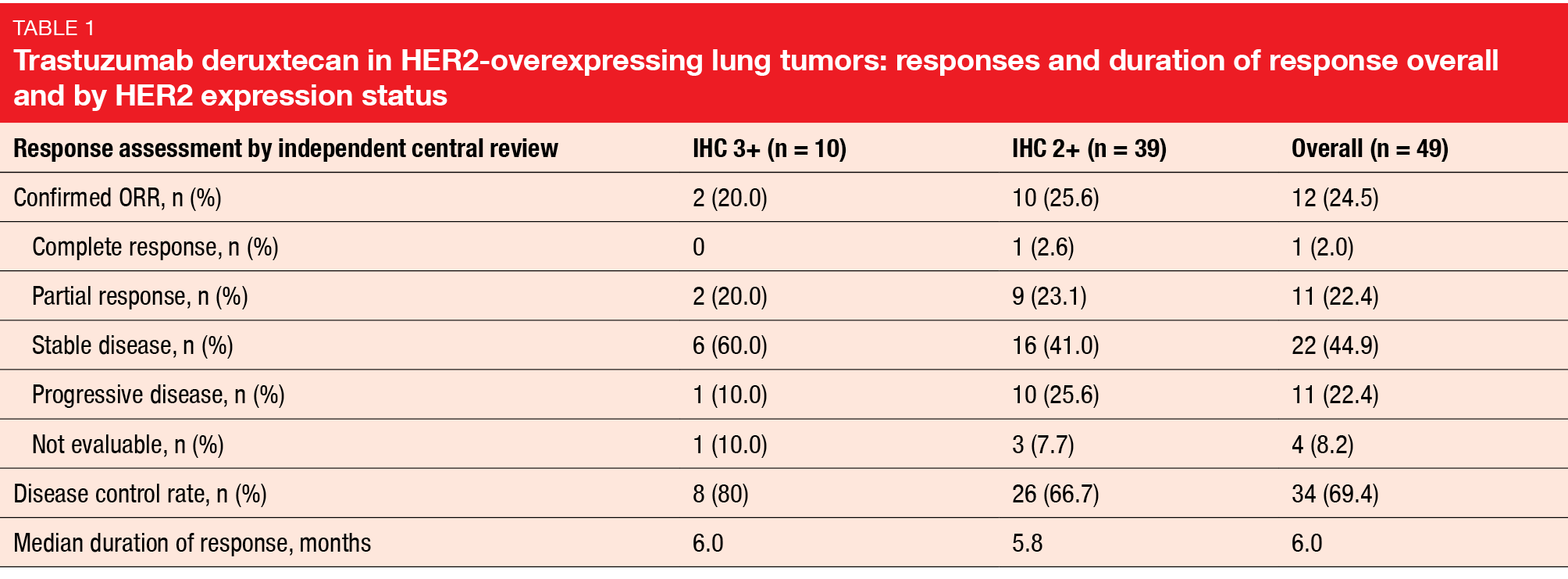
Somatic HER2 mutations are seen at relatively low frequencies across multiple tumor types [4]. Preclinical models demonstrated that a subset of these mutations result in constitutive kinase signaling, oncogenic transformation and enhanced tumor growth [5]. The open-label, multicenter, phase II DESTINY-Lung01 study was designed to assess the novel antibody-drug conjugate trastuzumab deruxtecan (T-DXd) in patients with HER2-positive, unresectable/metastatic non-squamous NSCLC who had relapsed on or were refractory to standard treatment. DESTINY-Lung01 consisted of Cohort 1 that included patients with HER2-overexpressing tumors (n = 49) and Cohort 2 comprising patients with HER2-mutated disease (n = 42). Patients in both cohorts received T-DXd 6.4 mg/kg 3-weekly. At WCLC 2020, Nakagawa et al. presented the interim results for Cohort 1 [6].
In this group of extensively pretreated patients with HER2-overexpressing NSCLC who had received a median of three lines of therapy, T-DXd showed evidence of anti-tumor activity. The overall ORR was 24.5 %, without any apparent difference by HER expression; for patients with IHC 3+ and IHC 2+, ORRs were 20.0 % and 25.6 %, respectively (Table). One patient developed CR (2.0 %), and disease control was achieved in 69.4 %. Responses lasted for a median of 6.0 months. Median PFS and OS were 5.4 months and 11.3 months, respectively.
Interstitial lung disease as a relevant issue
The safety profile was generally consistent with the observations from previous trials [7-11]. Drug-related treatment-emergent AEs (TEAEs) led to dose reductions in 32.7 %, while dose discontinuation due to the study drug became necessary in 12.2 %. Decreased neutrophil counts were the most common grade ≥ 3 TEAEs (20.4 %) and the main reason for dose reductions and interruptions (10.2 % each). Eight cases (16.3 %) of interstitial lung disease (ILD) occurred, with grade 1, 2 and 5 cases observed in 4.1 %, 6.1 %, and 6.1 %, respectively. Among the three fatalities reported in the context of ILD, pneumonitis was the cause of death in one patient. Overall, median time to onset of drug-related ILD was 64.5 days. The treatment was withdrawn in all cases, and steroids were used in the patients with grade 2 and 5 ILD. In their conclusion, the authors noted that the encouraging efficacy results noted in DESTINY-Lung01 support the ongoing exploration of T-DXd in patients with HER2-overexpressing NSCLC. ILD continues to be closely monitored and proactively managed in the study, with further investigation as more follow-up data are becoming available.
Smit et al. reported the findings for the Cohort 2 of the DESTINY-Lung01 trial that included 42 patients with HER2-mutated advanced NSCLC [12]. In this group, the ORR was 61.9 %, and disease control was obtained in 90.5 %. Responses proved durable, with median duration of response not having been reached at the time of the analysis. Median PFS was 14.0 months. As for Cohort 1, the safety profile generally corresponded with previous reports. No high-grade ILD events occurred. According to the authors, these data demonstrated the potential of T-DXd as a new treatment option in patients with HER2-mutated NSCLC, which is a patient population with a high unmet need. Enrollment in the HER2-mutated cohort was expanded with an additional 50 patients to better characterize T-DXd in this group and further support the ongoing clinical trial program.
Neratinib alone and in combination
The oral, irreversible TKI neratinib targets EGFR, HER2, and HER4 [13]. It has been shown to display clinical activity across a spectrum of HER2 mutations and tumor types, with sensitivity being both histology- and mutation-context–dependent [14]. Two international phase II trials assessed the activity of neratinib in patients with HER2-mutant lung cancers. PUMA-NER-4201 compared neratinib 240 mg/d (n = 17) in a randomized manner with neratinib 240 mg/d together with the mTOR inhibitor temsirolimus 8 mg/week (n = 43) in untreated or pretreated patients with stage IIIB/IV, HER2-mutated NSCLC. The open-label basket SUMMIT trial (PUMA-NER-5201) contained sequential open-label cohorts of patients with HER2-mutated lung cancers for which no curative therapy existed; they received neratinib alone (n = 26) or plus trastuzumab 8 mg/kg followed by 6 mg/kg 3-weekly (n = 52). Small in-frame insertions in exon 20 were the most frequent type of HER2 mutation in both studies (95 % and 67 % in PUMA-NER-4201 and SUMMIT, respectively). Li et al. presented the outcomes obtained in the trials [15].
The analyses indicated that single-agent neratinib has limited activity in HER2-mutated NSCLC. ORRs were 0 % and 4 % for the monotherapy cohorts included in 4201 and SUMMIT, respectively. The combinations with temsirolimus and trastuzumab gave rise to numerically higher ORRs of 14 % and 8 %, respectively. Here, responses proved durable in a subset of pretreated patients, lasting for up to 22.6 and 18.3 months, respectively. Median OS was longest with neratinib plus temsirolimus, at 15.1 months.
Diarrhea constituted the most common AE, with any-grade events occurring in > 80 % of patients across all cohorts. Grade ≥ 3 diarrhea was reported in up to 40 %. However, dose reductions and permanent treatment discontinuation were rare. Other AEs included nausea, vomiting, constipation and fatigue. The authors noted that additional novel combinations of neratinib with other HER2-directed therapies in HER2-mutant NSCLC are being considered.
ARIA
In ALK-positive NSCLC patients, the detection of resistance mechanisms to ALK TKI therapy might help to select the subsequent treatment. The ARIA study investigated the activity of next-generation ALK TKIs based on the presence of ALK resistance mutations in circulating tumor DNA (ctDNA) obtained by liquid biopsy [16]. This analysis included 58 patients at 9 European sites who had ALK-positive, advanced NSCLC pretreated with first- and/or second-generation ALK TKIs. Liquid biopsy was collected immediately before the initiation of brigatinib or lorlatinib therapy. The activity of these two drugs was evaluated based on three ctDNA molecular groups:
- those with ALK mutations (only 1 mutation: single; ≥ 2 mutations: complex)
- those with non-ALK mutations
- those without detectable mutations.
Among the 58 evaluable patients, 16 and 42 received brigatinib and lorlatinib, respectively. In these subgroups, 94 % and 74 % had previously been treated with crizotinib; the respective percentages for previous second-generation ALK TKI treatment were 69 % and 98 %. Most patients showed more than two metastatic sites, with intracranial lesions present in 88 % and 71 %, respectively.
Overall, ALK mutations were detected in 16 (28 %) patients; 9 of these were single, and 7 were complex. Other mutations were seen in 17 %, and 55 % of patients showed no mutations. The ALK mutations included a wide spectrum, with G1202R occurring most commonly, followed by G1269A and F1174L (Figure 2). Per sample, 1–6 mutations were found. Five of the 16 patients with ALK mutations had previously received alectinib, another 5 had been treated with ceritinib, and 6 had received brigatinib.
Figure 2: Subtypes of ALK mutations as assessed by liquid biopsy
Outcomes according to ALK resistance mutations
The subsequent treatment with lorlatinib demonstrated activity regardless of the ctDNA molecular groups. Median PFS was comparable across patients with ALK mutations (n = 13; 6.5 months), other mutations (n = 7; 7.6 months), and no mutations (n = 22; 7.3 months). ORRs amounted to 46 %, 71 %, and 23 %, respectively, and ORRs in the CNS were 56 %, 60 %, and 67 %, respectively. Median OS in the three groups was 62.6 months, 45.0 months, and had not been reached yet, respectively.
In contrast, outcomes observed for brigatinib in the ALK-mutated group were poor, although conclusions pertaining to this cohort are limited as it only comprised three individuals and patient numbers were low in general. Median PFS was 3.5 months compared to 6.2 months in the group with other mutations (n = 3) and 8.1 months in those without any mutations (n = 10). ORRs were 0 %, 67 %, and 22 %, respectively, and CNS responses occurred in 0 %, 100 %, and 50 %, respectively. Median OS amounted to 38.4 months, 62.6 months, and had not been reached yet, respectively. Summarizing these results, the authors emphasized that the recent upfront use of second-generation TKI therapy calls for similar studies to confirm if ctDNA might be a biomarker for guiding sequential therapy.
REFERENCES
- Biernacka A et al., The potential utility of re-mining results of somatic mutation testing: KRAS status in lung adenocarcinoma. Cancer Genet 2016; 209(5): 195-198
- Hong DS et al., KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med 2020; 383: 1207-1217
- Li BT et al., CodeBreaK 100: registrational phase 2 trial of sotorasib in KRAS p.G12C mutated non-small cell lung cancer. WCLC 2020, PS01.07
- Cousin S et al., Targeting ERBB2 mutations in solid tumors: biological and clinical implications. J Hematol Oncol 2018; 11(1): 86
- Bose R et al., Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov 2013; 3(2): 224-237
- Nakagawa K et al., Trastuzumab deruxtecan in HER2-overexpressing metastatic non-small cell lung cancer (NSCLC): interim results of DESTINY-Lung01. WCLC 2020, OA04.05
- Tsurutani J et al., Targeting HER2 with trastuzumab deruxtecan: a dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discov 2020; 10(5): 688-701
- Tamura K et al., Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol 2019; 20(6): 816-826
- Modi S et al., Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 2020; 382(7): 610-621
- Modi S et al., Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol 2020; 38(17): 1887-1896
- Shitara K et al., Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol 2019; 20(6): 827-836
- Smit EF et al., Trastuzumab deruxtecan in HER2-mutated metastatic non-small cell lung cancer (NSCLC): interim results of DESTINY-Lung01. WCLC 2020, MA11.03
- Wissner A & Mansour TS, The development of HKI-272 and related compounds for the treatment of cancer. Arch Pharm Chem Life Sci 2008; 341(8): 465-477
- Hyman DM et al., HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 2018; 554(7691):189-194
- Li BT et al., Neratinib-based combination therapy in HER2-mutant lung adenocarcinomas: findings from two international phase 2 studies. WCLC 2020, FP14.15
- Mezquita L et al., The ARIA study: Activity of next-generation ALK TKIs based on ALK Resistance mutations detected by liquid biopsy in ALK positive NSCLC patients. WCLC 2020, P84.01
© 2020 Springer-Verlag GmbH, Impressum
More posts
Interview: Lung cancer screening: hurdles in daily routine and in the research laboratory
Interview: Lung cancer screening: hurdles in daily routine and in the research laboratory