Established targeted agents taking root in the HER2-positive setting
HER2 aberrations in lung cancer are being increasingly identified due to the use of sensitive testing procedures, such as multiplexed testing and next-generation sequencing. Mutations of the HER2 gene need to be distinguished from HER2 amplifications and HER2 protein overexpression. In contrast to breast and gastric cancer, HER2 overexpression in NSCLC does not always occur with HER2 amplification, while amplifications and HER2 mutations are generally mutually exclusive [1]. As the various types of aberrations represent distinct molecular targets, tumours need to be precisely characterised at the molecular level prior to treatment decisions. To date, however, there is no approved treatment for patients with HER2-positive NSCLC.
Trastuzumab after progression on EGFR TKIs
Patients with EGFR-mutated NSCLC who develop EGFR TKI resistance tend to undergo activation of a HER2 bypass track. HER2 overexpression and HER2 amplification are found in up to 17 % of these tumour biopsies [2, 3]. A single-arm, open-label, phase II trial determined whether 24 patients with EGFR-mutated, non-squamous, stage IV lung cancer who showed HER2 overexpression after progression on EGFR TKI monotherapy derived any benefit from the HER2 antibody trastuzumab (2 mg/ kg weekly, after a loading dose of 4 mg/ kg) in combination with paclitaxel 60 mg/m2 [4].
Trastuzumab plus paclitaxel was well tolerated and induced durable tumour responses in a considerable proportion of patients. The objective response rate was 46 %, and 63 % achieved disease control at 6 weeks. Median survival in the intention-to-treat group was 3 years. The findings implied a positive correlation between response rates/ disease control rates and both HER2 expression levels and HER2 gene copy numbers. However, the regimen appeared to have limited activity against brain metastases, as isolated brain progression with extra-cerebral stable disease or partial response was observed in 21 % of cases. Extra-cerebral responses occurred in 58 %.
Results with T-DM1 in HER2-mutated tumours
HER2 mutations have been identified as oncogenic drivers in approximately 3 % of lung cancers [5]. A phase II basket trial explored the antibody-drug conjugate ado-trastuzumab, also known as T-DM1, in patients with HER2-amplified and HER2-mutant advanced solid cancers, at 3.6 mg/kg every 3 weeks until disease progression [6]. T-DM1 consists of trastuzumab and the cytotoxic agent emtansine, and it has already been approved for the treatment of HER2-positive breast cancer. After HER2-targeted binding, the emtansine released in the tumour cell induces apoptosis.
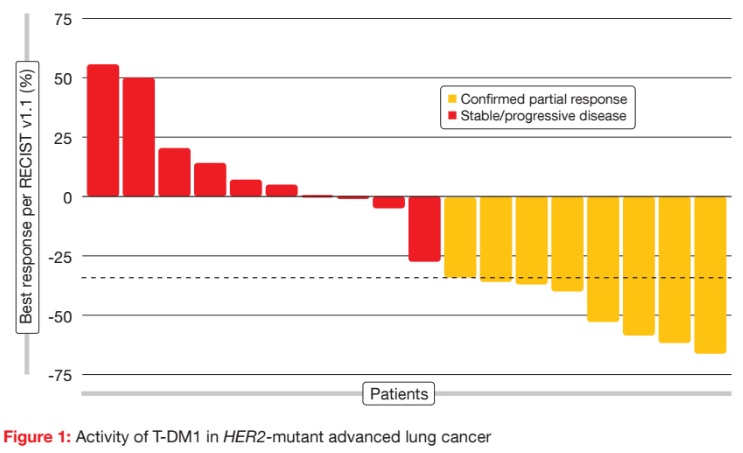
The basket trial contained separate cohorts for HER2-amplified lung cancers and HER2-mutant lung cancers. At the ASCO Congress, the results were presented for the HER2-mutant cohort, which comprised 18 patients. T-DM1 was active and well tolerated in this group. For the primary endpoint, the ORR was 44 %, with eight patients responding (Figure 1). There was no relationship between prior therapy and response, as six of the eight responders were heavily pre-treated, including prior HER2-targeted therapy. Responses lasted for a median of 5 months. Median PFS was 4 months. Treatmentrelated AEs were mainly rated as grades 1 and 2. AEs did not require any dose reductions or treatment discontinuations. According to the molecular analysis, these responses occurred across various mutation subtypes. HER2 protein levels were low in the eight responders, who therefore benefited from T-DM1 treatment even though their HER2 expression was not pronounced. These results justify a confirmatory multicentre study for patients with HER2-mutated lung cancers.
T-DM1 and HER2 overexpression
A study presented by Stinchcombe et al. was the first trial to report on the clinical activity of T-DM1 in HER2-overexpressing metastatic NSCLC [7]. HER2 overexpression was used as an inclusion criterion here. Forty patients with lung cancer of any histology that was HER2– positive according to central prospective immunohistochemistry (IHC) testing received T-DM1 3.6 mg/kg every 3 weeks until disease progression. The study included two cohorts with 20 patients in each: those with HER2 IHC 2+ staining, and those with HER2 IHC 3+ staining. Patients had received at least one prior platinum-based chemotherapy. ORR was defined as the primary endpoint.
Four patients in the IHC 3+ group responded to T-DM1 treatment (20 %), three of whom responded rapidly. The median duration of response was 7.3 months. On the other hand, no objective responses occurred in the IHC 2+ cohort, although several patients achieved stable disease (SD) for 6 months, and one patient had SD for over 20 months. Median PFS in the IHC 3+ and IHC 2+ cohorts was 2.7 and 2.6 months, respectively, and median OS was 12.1 and 12.2 months, respectively. No new safety signals were observed. According to the exploratory biomarker analysis, a higher percentage of patients in the IHC 3+ cohort, compared to the IHC 2+ cohort, showed HER2-amplified tumours, high gene copy numbers, and HER2 mRNA. Additional investigations into improved detection of HER2 amplification and other biomarkers might help to refine the patient population that is most likely to benefit from T-DM1 in future studies.
What can afatinib do?
To explore the effects of the irreversible ErbB family blocker afatinib in the HER2-positive setting, Lai et al. retrospectively reviewed clinicopathological data from patients with metastatic, HER2-mutated lung cancer who were treated with afatinib across seven institutions between 2009 and 2017 [8]. The primary endpoint was investigator-assessed overall response. Before afatinib therapy, the patients had received a median of two lines of treatment.
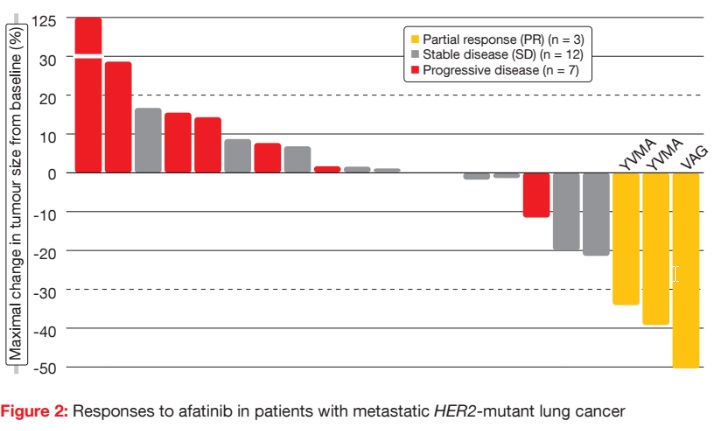
Partial responses occurred in three of the 27 patients (11 %; Figure 2), with a mean duration of response of 6 months. Two of these patients had the YVMA mutation, and one patient had the VAG mutation. SD was observed in 16 cases. Five patients received afatinib treatment for more than 6 months. Of these, four had the YVMA mutation, and one had the S310F mutation. One patient with SD as best response remained on afatinib beyond 30 months. Median OS was 23 months.
Overall, these findings provide data supporting the use of afatinib in HER2– mutant lung cancers. The authors noted that afatinib can still be considered as a treatment option when patients have previously progressed on HER2-targeted therapies. Two phase II trials investigating afatinib in the HER2-positive setting are ongoing: the ETOP NICHE trial in Europe, and the NCI-MATCH trial in the USA.
ETOP NICHE
The primary objective of the single-arm, phase II ETOP NICHE trial is to determine the disease control obtained with afatinib in pre-treated patients with stage IIIB/IV NSCLC harbouring HER2 exon 20 mutations [9]. The primary endpoint is complete or partial response, or SD, for at least 12 weeks. Thirteen patients are participating in this trial.
Overall, afatinib therapy resulted in a lower disease control rate than expected in this Simon’s two-stage design trial [10]. At the time of the interim analysis, five out of the first nine patients failed to maintain SD for 12 weeks. Accrual was closed, because the stopping threshold had been reached. The treatment and follow-up of the enrolled patients continued based on the judgement of the treating physicians.
Although this approach failed to meet the defined criteria for further clinical testing, signs of activity were observed in the full analysis set. Median PFS was 15.9 months, and the 12-weeks PFS rate was 53.8 %. Descriptive molecular analysis of the tumours suggests that patients with A775-G776insYV HER2 mutations experienced prolonged disease stabilisation, but the small number of patients limits any clear pattern of association with the molecular data.
References:
- Li BT et al., HER2 amplification and HER2 mutation are distinct molecular targets in lung cancers. J Thorac Oncol 2016; 11(3): 414-419
- Yu HA et al., Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013; 2240-2247
- Altavilla GA et al., Occurrence of HER2 amplification in EGFR-mutant lung adenocarcinoma with acquired resistence to EGFR-TKIs. J Clin Oncol 2013; suppl; abstr 8047
- de Langen A et al., Trastuzumab and paclitaxel in patients with EGFR mutated NSCLC that express HER2 after progression on EGFR TKI treatment. ASCO 2017, abstract 9042
- Kris MG et al., Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014; 311(19): 1998-2006
- Li BT et al., Ado-trastuzumab emtansine in patients with HER2 mutant lung cancers: results from a phase II basket trial. ASCO 2017, abstract 8510
- Stinchcombe T et al., Efficacy, safety, and biomarker results of trastuzumab emtansine (TDM1) in patients with previously treated HER2- overexpressing locally advanced or metastatic non-small cell lung cancer (mNSCLC). ASCO 2017, abstract 8509
- Lai W-C V et al., Afatinib in patients with metastatic HER2-mutant lung cancers: An international multicenter study. ASCO 2017, abstract 9071 9 Smit EF et al., A single-arm phase II trial of afatinib in pretreated patients with advanced NSCLC harbouring a HER2 mutation. The ETOP NICHE trial. ASCO 2017, abstract 9070
More posts
Anti-angiogenic and immunotherapeutic approaches in mesothelioma
Malignant pleural mesothelioma (MPM) is a rare tumour that is often diagnosed at an advanced stage. Limited efficacy of the available therapies contributes to the generally poor prognosis for MPM patients. Since 2003, the only approved regimen for MPM treatment has been chemotherapy with pemetrexed and cisplatin, with median survival of approximately 12 months.
Interview: Lung cancer in China: hurdles and progress
Lung cancer is a considerable issue in China. Every year, we have 700,000 new cases. There is a need to perform clinical trials and to launch innovative drugs. With regard to the introduction of targeted therapies, China lags 3 to 4 years behind when compared to the western countries. Two months ago, the EGFR TKI afatinib was launched, offering Chinese patients with EGFR-mutant lung cancer an effective treatment option.
Real-world utility of ctDNA NGS to identify matched targeted therapy
Liquid biopsy for plasma circulating tumour DNA (ctDNA) next generation sequencing (NGS) is a rapidly evolving science. Plasma ctDNA assays are now commercially available, and are increasingly adopted in the community with a paucity of evidence-based guidance on timing and value of this test. Sabari et al. sought to determine the feasibility and utility of plasma ctDNA NGS to identify matched targeted therapy in a real-world clinical setting.
Further defining the optimal use of immune checkpoint inhibitors
As the anti-PD-1 antibody nivolumab is known to induce deep and durable responses in a subset of lung cancer patients, this agent was investigated in the neoadjuvant setting, which is an area of unmet need. There have been no advances in systemic treatment of resectable lung cancer since 2004. Chaft et al. hypothesised that neoadjuvant nivolumab treatment might induce immunity against micrometastases.
Established targeted agents taking root in the HER2-positive setting
HER2 aberrations in lung cancer are being increasingly identified due to the use of sensitive testing procedures, such as multiplexed testing and next-generation sequencing. Mutations of the HER2 gene need to be distinguished from HER2 amplifications and HER2 protein overexpression. In contrast to breast and gastric cancer, HER2 overexpression in NSCLC does not always occur with HER2 amplification, while amplifications and HER2 mutations are generally mutually exclusive.
Diagnostics of EGFR-mutant disease: biomarkers with significant clinical implications
The clinical relevance of additional genetic alterations in advanced EGFR-mutant NSCLC is not clear. Blakely et al. hypothesised that co-occurring genomic alterations in cancer-related genes can cooperate with the mutant EGFR to drive de-novo resistance to EGFR TKI treatments. The investigators performed targeted exome sequencing of plasma cell-free DNA (cfDNA) in 86 samples collected from 81 patients with known clinical history.