Reducing the danger that arises from the CNS as a site of progression
Brain metastases and leptomeningeal disease represent major clinical challenges in the management of patients with NSCLC. They are generally associated with poor prognosis, and their treatment is difficult due to the paucity of effective therapeutic options. Moreover, patients with CNS lesions are frequently excluded from clinical trials. Progress in this area is therefore slow, and more treatments are urgently needed.
NVALT-11: prophylactic irradiation
In patients with stage III NSCLC, the brain is the most important site of treatment failure. Prophylactic cranial irradiation (PCI) has been shown to reduce the incidence of brain metastases in patients with NSCLC, but the exact value of PCI in stage III NSCLC patients receiving contemporary chemoradiation schedules with or without surgery remains uncertain. The investigators in the phase III NVALT-11 trial hypothesised that PCI reduces the incidence of symptomatic CNS lesions in radically treated stage III NSCLC [1].
Patients underwent concurrent or sequential chemoradiation, or concurrent chemoradiation with or without induction chemotherapy and resection before trial inclusion. If they had WHO performance status 0-2 after 2 to 3 weeks of completion of radical therapy, plus no clinical signs of disease progression, they were randomised to either PCI (36 Gy in 18 fractions; 30 Gy in 12 fractions; 30 Gy in 10 fractions) or observation. PCI was started within 4 weeks of completion of radical therapy. The proportion of patients who developed symptomatic brain metastases was defined as the primary endpoint. While 86 patients received PCI, 88 made up the observation cohort.
Indeed, a smaller proportion of patients treated with PCI developed symptomatic brain metastases compared to observation (4.6 % vs. 28.4 %; p < 0.001). PCI also significantly increased the time to development of symptomatic brain metastases (HR, 0.25; p = 0.001). This was also true for the time to development of brain metastases irrespective of symptoms (HR, 0.26). However, PCI did not prolong OS, and the experimental treatment significantly decreased global quality of life 3 months after PCI, compared to observation (p = 0.02). Thereafter, no differences in quality of life were noted. Time to all neurological symptoms did not differ across these study groups.
Afatinib penetration into brain metastases
Up to 40 % of patients with EGFRmutation- positive NSCLC develop brain metastases over the course of their disease [2]. Failure of drugs to penetrate the blood–brain barrier (BBB) can be a major reason for treatment failure in brain disease. The investigator-initiated CamBMT1 trial is currently exploring the extent to which the small-molecule, irreversible, ErbB family blocker afatinib crosses the BBB, and is attempting to answer the question of whether the delivery of afatinib into brain metastases can be improved by radiotherapy, as it has been suggested that low-dose radiotherapy might disrupt the BBB. Patients with operable brain metastases from breast or lung origin are participating in this window-ofopportunity study that has a two-phase design. The main trial, which is currently recruiting patients, is a three-arm randomised phase II study to compare preoperative afatinib alone with afatinib plus a single fraction of radiotherapy administered as either 2 Gy or 4 Gy. This was preceded by a safety run-in phase Ib trial to test afatinib over 11 days. On day 10, a single fraction of radiotherapy at either 2 Gy (Arm A) or 4 Gy (Arm B) was applied, because afatinib was expected to be at steady-state levels at that time. Neurosurgery was performed on day 12.
At the ASCO Congress, Baird et al. presented the phase Ib results [3]. This part of the trial used an accelerated titration design with three pre-planned dose levels of afatinib. In each of the 2 Gy and 4 Gy cohorts, 1 patient was treated at the 20 mg dose level, 1 patient at 30 mg, and 3 patients at 40 mg, for a total population of 10 patients. Six patients had brain metastases from lung cancer origin. The objective of the phase Ib study was to establish feasibility and the recommended phase II dose for this combination with radiotherapy.
Distinct cerebral accumulation
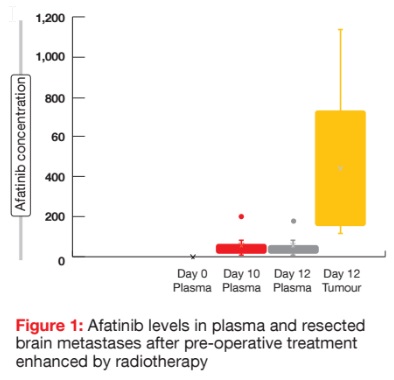
The recommended phase II dose of afatinib with a single 2 or 4 Gy fraction of radiotherapy given pre-operatively was established as 40 mg daily. Pharmacokinetic results for both cohorts combined showed that afatinib concentrations in resected brain lesions were on average more than 15-fold higher than those in the plasma. On day 12, the median plasma and tumour afatinib concentrations were 22.7 ng/ml and 405 ng/g, respectively (Figure 1). The treatments were well tolerated, and no dose-limiting toxicities occurred.
As the authors conceded, the number of patients included in this trial was small, and it is not yet certain that high afatinib concentrations in brain metastases might have been achieved without radiotherapy. However, preclinical studies in rats have suggested that afatinib accumulates in tissues, although afatinib concentrations in the rat brain were 20-fold to 50-fold lower than in other tissues, which suggests an effect of the BBB [4]. Moreover, the afatinib concentration in normal brain tissue of rats was only 3-fold to 4-fold higher compared to the plasma levels. Phase II of the CamBMT1 study, which is presently ongoing, will provide direct determination of the enhancement of delivery of afatinib into brain metastases by radiotherapy.
Intracranial activity of osimertinib in AURA3
The AURA3 trial demonstrated significantly greater efficacy of osimertinib 80 mg/d compared to platinum-based chemotherapy in the T790M-positive setting following progression after first-line EGFR TKI treatment [5]. Based on the AURA3 data, Mok et al. presented the first comparative evidence of osimertinib activity in CNS metastases from a randomised phase III study [6]. Patients with stable asymptomatic brain lesions were eligible for AURA3.
Two analyses were performed. The first one was the ‘CNS full analysis set’; i.e., patients with measurable and/ or non-measurable CNS disease. These represented 28 % of the overall population. Here, 75 and 41 subjects received osimertinib and platinumpemetrexed chemotherapy, respectively. The endpoint for this full analysis set was CNS PFS. The second cohort included only those with at least one measurable CNS lesion (11 % of the overall population). Thirty and 16 patients in this group were treated with osimertinib and chemotherapy, respectively. CNS objective response rate and CNS duration of response constituted the objectives for this cohort, which was designated as the ‘CNS evaluable for response set’.
Longer CNS PFS and higher CNS ORR
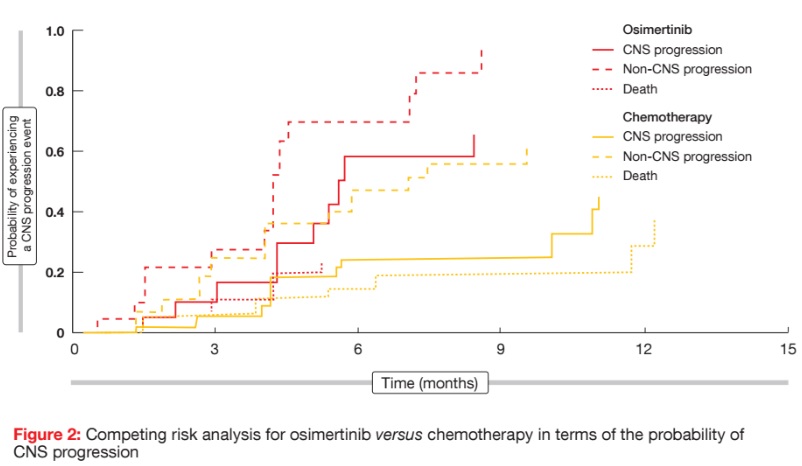
In this evaluable for the response set, CNS ORRs were 70 % and 31 % for patients treated with osimertinib and chemotherapy, respectively (odds ratio, 5.13; p = 0.015). Responses lasted 8.9 and 5.7 months, respectively. CNS disease control was achieved in 93 % versus 63 %. CNS responses to osimertinib started at 6.1 weeks, which corresponded to the first radiological evaluation. The effects of treatment were observed regardless of prior brain radiotherapy status. In osimertinib-treated patients, CNS ORR was 64 % versus 34 % in those who had received radiotherapy within 6 months of randomisation versus those without prior brain radiation or radiotherapy ≥ 6 months before randomisation. For chemotherapy, this was 22 % versus 16 %. The majority of patients experienced shrinkage of brain metastases, although responses appeared to be more frequent and deeper in the osimertinib group. The full analysis set derived a statistically significant PFS benefit from osimertinib treatment, as compared to chemotherapy (11.7 vs. 5.6 months; HR, 0.32; p = 0.004). According to a competing risk analysis for this patient cohort, the probability of experiencing a CNS progression event was lower for osimertinib than for chemotherapy at both 3 and 6 months (Figure 2). At 6 months, the cumulative incidence of brain metastases was 11.5 % vs. 28.2 % for osimertinib and chemotherapy, respectively. A similar reduction of risk occurred in terms of the pattern of non-CNS progression. Furthermore, encouraging activity was seen for patients with leptomeningeal disease; here, 4 out of 7 subjects experienced responses, with two achieving complete remission.
BLOOM trial: osimertinib in leptomeningeal disease
Yang et al. presented updated data from the phase I BLOOM study that investigated osimertinib 160 mg/d in patients with advanced, EGFR-mutationpositive NSCLC who had progressed on prior EGFR TKI therapy and showed leptomeningeal disease [7]. Patients were recruited either into a T790Mpositive cohort or a T790M-unselected cohort. The results presented at ASCO referred to the unselected population only (n = 21), as those from the T790Mpositive cohort were not mature yet.
Overall leptomeningeal responses by investigator assessment were found in 43 % of these 21 patients, and median duration of response was 18.9 months. All of the patients underwent neurological examinations. Of the 11 patients with ‘normal’ baseline assessment, 10 had no change in neurological findings, and one worsened (change from ‘normal’ to ‘mildly abnormal’). Of the 10 patients with ‘abnormal’ baseline neurological assessment, seven experienced improvement. Data were recorded as ‘missing’ for 3 patients. According to the evaluation of cerebrospinal fluid (CSF) after the exclusion of 1 patient, 30 % of patients had confirmed CSF response. Pharmacokinetic analysis revealed that osimertinib 160 mg/d penetrates the BBB, which resulted in mean CSF osimertinib concentration of 7.5 nM. The CSF:free plasma ratio was 16.4 %. The safety and tolerability profile matched the known profile of osimertinib 160 mg/d. Overall, these data suggest that osimertinib has the potential for use in patients with leptomeningeal disease, although further evaluation in larger clinical studies is needed to confirm these findings.
References:
- Groen HJM et al., Prophylactic cranial irradiation (PCI) versus observation in radically treated stage III non-small cell lung cancer (NSCLC): a randomized phase III study (NVALT-11). ASCO 2017, abstract 8502
- Rangachari D et al., Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015; 88(1): 108-111
- Baird R et al., Cambridge Brain Mets Trial 1 (CamBMT1): A proof of principle study of afatinib penetration into cerebral metastases for patients undergoing neurosurgical resection, combined with low-dose, targeted radiotherapy – Phase 1b results. ASCO 2017, abstract 2008
- CHMP assessment report EMA/491185/2013
- Mok TS et al., Osimertinib or platinumpemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017; 376(7): 629-640
- Mok T et al., CNS response to osimertinib in patients with T790M-positive advanced NSCLC: data from a randomized phase III trial (AURA3). ASCO 2017, abstract 9005
- Yang J C-H et al., Osimertinib for patients with leptomeningeal metastases from EGFRmutant non-small cell lung cancer: updated results from the BLOOM study. ASCO 2017, abstract 2020
More posts
Anti-angiogenic and immunotherapeutic approaches in mesothelioma
Malignant pleural mesothelioma (MPM) is a rare tumour that is often diagnosed at an advanced stage. Limited efficacy of the available therapies contributes to the generally poor prognosis for MPM patients. Since 2003, the only approved regimen for MPM treatment has been chemotherapy with pemetrexed and cisplatin, with median survival of approximately 12 months.
Interview: Lung cancer in China: hurdles and progress
Lung cancer is a considerable issue in China. Every year, we have 700,000 new cases. There is a need to perform clinical trials and to launch innovative drugs. With regard to the introduction of targeted therapies, China lags 3 to 4 years behind when compared to the western countries. Two months ago, the EGFR TKI afatinib was launched, offering Chinese patients with EGFR-mutant lung cancer an effective treatment option.
Real-world utility of ctDNA NGS to identify matched targeted therapy
Liquid biopsy for plasma circulating tumour DNA (ctDNA) next generation sequencing (NGS) is a rapidly evolving science. Plasma ctDNA assays are now commercially available, and are increasingly adopted in the community with a paucity of evidence-based guidance on timing and value of this test. Sabari et al. sought to determine the feasibility and utility of plasma ctDNA NGS to identify matched targeted therapy in a real-world clinical setting.
Further defining the optimal use of immune checkpoint inhibitors
As the anti-PD-1 antibody nivolumab is known to induce deep and durable responses in a subset of lung cancer patients, this agent was investigated in the neoadjuvant setting, which is an area of unmet need. There have been no advances in systemic treatment of resectable lung cancer since 2004. Chaft et al. hypothesised that neoadjuvant nivolumab treatment might induce immunity against micrometastases.
Established targeted agents taking root in the HER2-positive setting
HER2 aberrations in lung cancer are being increasingly identified due to the use of sensitive testing procedures, such as multiplexed testing and next-generation sequencing. Mutations of the HER2 gene need to be distinguished from HER2 amplifications and HER2 protein overexpression. In contrast to breast and gastric cancer, HER2 overexpression in NSCLC does not always occur with HER2 amplification, while amplifications and HER2 mutations are generally mutually exclusive.
Diagnostics of EGFR-mutant disease: biomarkers with significant clinical implications
The clinical relevance of additional genetic alterations in advanced EGFR-mutant NSCLC is not clear. Blakely et al. hypothesised that co-occurring genomic alterations in cancer-related genes can cooperate with the mutant EGFR to drive de-novo resistance to EGFR TKI treatments. The investigators performed targeted exome sequencing of plasma cell-free DNA (cfDNA) in 86 samples collected from 81 patients with known clinical history.