Anti-PD-1 compounds targeting MSI-H/dMMR tumors
Keynote-158: an update of pembrolizumab in MSI-H/dMMR solid tumors
Accurate and timely repair of DNA is essential for maintaining genetic stability [1]. Microsatellites are repetitive DNA sequences and particularly prone to replication errors that are normally repaired by the mismatch repair system [2]. Mismatch repair-deficient tumors (dMMR) harbor many mutations in microsatellites, resulting in high levels of microsatellite instability (MSI-H) [3]. MSI-H/dMMR tumors are immunogenic, triggering the upregulation of immune checkpoint proteins such as programmed cell death protein 1 (PD-1); those tumors have been recently shown to be responsive to PD-1 blockade [4, 5].
Based on the findings of the KEYNOTE-158 study, the anti-PD-1 antibody pembrolizumab was the first immune checkpoint inhibitor (CPI) approved in 2017 for the treatment of unresectable or metastatic MSI-H/dMMR solid tumors following progression on prior standard therapy [3, 6]. KEYNOTE-158 trial (NCT02628067) was a multi-cohort, open-label, non-randomized phase II study which evaluated pembrolizumab in pan-tumor patients enrolled from 81 study centers across 21 countries worldwide [7]. Among all cohorts, the study demonstrated a clinical benefit for 233 previously treated MSI-H/dMMR patients with solid tumors: an overall response rate (ORR) – the primary endpoint according to RECIST v1.1 and assessed by independent central radiologic review – of 34.3 %, a median progression free survival (PFS) of 4.1 months, and a median overall survival (OS) of 23.5 months [3].
At the virtual ASCO 2021 meeting, Maio et al. presented an additional 22-month follow-up of the KEYNOTE-158 trial in MSI-H patients with advanced non-colorectal solid tumors (cohort K) [8]. MSI-H/dMMR status was evaluated locally from a tumor tissue sample and defined as ≥ 1 of 4 MMR proteins absent by immunohistochemistry (IHC) or as ≥ 2 allelic loci size shifts of 5 microsatellite markers by polymerase chain reaction (PCR). Eligible patients received pembrolizumab (200 mg once every three weeks) for up to two years or until disease progression, unacceptable toxicity, investigator decision, or withdrawal of consent. Secondary endpoints included duration of response (DoR), PFS, OS, safety, and tolerability.
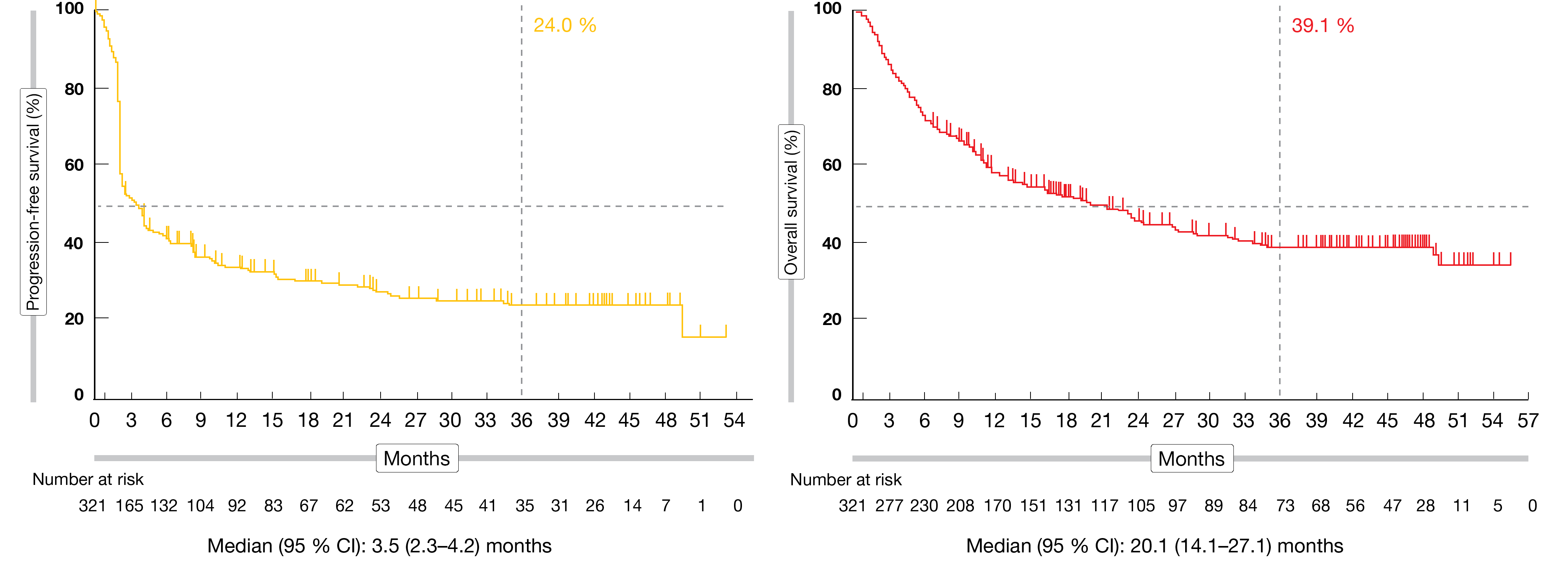
The cohort K included multiple tumor types, like endometrial (22.5 %), gastric (14.5 %), small intestine (7.4 %), ovarian (7.1 %), cholangiocarcinoma (6.3 %), and pancreatic cancer (6.3 %). Out of the 351 enrolled patients in this cohort, 27 (8.4 %) had a confirmed complete response (CR), 72 (22.4 %) had a partial response (PR), and 61 (19.0 %) had a stable disease (SD). The ORR among the 321 eligible patients was 30.8 % (95 % CI, 25.8-36.2). Overall, 70.1 % of patients had a continued response at 36 months. The median PFS was 3.5 months (95 % CI, 2.3-4.2) and the estimated 36-month PFS rate attained 24.0 %. At the time of analysis, median OS was 20.1 % (95 % CI, 14.1-27.1) and the estimated 36-month OS rate amounted to 39.1 % (Figure 1).
The safety profile was consistent with previous analyses. Treatment-related adverse events (TRAEs) occurred in 64.7 % of patients; 12.0 % experienced TRAEs grade ≥ 3. The most common AEs in 5 % or more of patients were pruritus (14.5 %), fatigue (12.3 %), and diarrhea (11.7 %). Immune mediated AEs and infusion reactions occurred in 20.2 % of patients (in 4.8 % with grade ≥ 3) and led to death in two patients because of myocarditis and Guillain-Barré syndrome.
Pembrolizumab demonstrated a maintained clinical benefit and a manageable safety profile in a heavily pretreated study population with advanced MSI-H/dMMR non-colorectal cancer.
Figure 1: Updated PFS and OS Kaplan-Meier curves of KEYNOTE-158 (cohort K)
Tislelizumab: a novel treatment option for solid tumors
Tislelizumab is a uniquely designed humanized immunoglobulin G4 (IgG4) monoclonal antibody with high affinity and binding specificity for PD-1 [9]. This anti-PD-1 antibody was engineered to minimize the binding to the Fcγ receptor (FcγR) on macrophages, which might be a potential strategy to circumvent resistance to anti-PD-1 therapy through the abrogation of the antibody-dependent cellular phagocytosis (ADCP) [9]. In early phase studies, Tislelizumab showed a good tolerability and antitumor activity against multiple solid tumors [10-12]. In 2019, tislelizumab was approved in China for patients with relapsed or refractory Hodgkin´s lymphoma after at least a second-line chemotherapy [13]. Tislelizumab is being currently evaluated in several global pivotal trials in a wide range of tumors, including esophageal squamous cell carcinoma, hepatocellular carcinoma, and non-small cell lung cancer [13].
This single-arm, non-randomized, open-label, multicenter, phase II study investigated the efficacy and safety of tislelizumab in patients with previously treated, locally advanced unresectable or metastatic MSI-H/dMMR solid tumors, including colorectal cancer (CRC) (NCT03736889) [14]. Adult Chinese patients with at least one measurable lesion according to RECIST v1.1, who received or refused prior cancer therapy regimen(s) for advanced disease, were treated with tislelizumab (200 mg intravenously three-weekly) until disease progression, unacceptable toxicity, or withdrawal. The primary endpoint was ORR as assessed by independent review committee (IRC); optionally, patients were able to continue tislelizumab monotherapy after an investigator-assessed radiological progression. Time to response (TTR), disease control rate (DCR), DoR, as well as safety and tolerability, were the secondary study endpoints.
Among the 80 patients enrolled, 74 (median age, 53 years; range, 19-75) were included in the primary efficacy analysis set. In total, 56.8 % of them were male and almost all patients had metastatic disease. Overall, 62.2 % of patients suffered from CRC, while 17.6% had endometrial cancer, 10.8 % gastric/gastroesophageal junction (GI/GEJ) cancer, 4.1 % small bowel adenocarcinoma and 5.4 % another type of cancer. The median number of prior therapy regimens was two (range, 0-7). Among all tumor types, tislelizumab monotherapy resulted in an ORR of 45.9 % (95 % CI, 34.3-57.9; p<0.0001) after a median follow-up of 11.8 months. A high rate of disease control (DCR, 71.6 %) was shown with tislelizumab treatment across all tumor entities (4 CRs, 30 PRs and 19 SDs). The clinical benefit rate was 52.7 % and 71.6 % of patients reached a disease control. The observed ORR amounted 39.1 % (95 % CI, 25.1-54.6; 2 CRs, 16 PRs and 15 SDs) in CRC patients (n = 46) and 57.1 % (95 % CI, 37.2-75.5; 2 CRs, 14 PRs and 4 SDs) in non-CRC patients (n = 28). A reduction of target lesion size compared to baseline was reported in seven out of eight enrolled tumor types (Figure 2). Median DoR, PFS and OS have not been reached yet. For all analyzed tumors, 12-month PFS and OS rates were respectively 59.3 % (95 % CI, 46.2-70.2) and 75.3 % (95 % CI, 62.6-84.2). No disease progression was reported in the 34 responders, while 33 responders were still on treatment and one patient started a new anti-cancer therapy at the time of analysis.
In the safety population (n = 80), treatment-emergent adverse events (TEAEs) grade ≥ 3 occurred in 47.5% of patients, including laboratory abnormalities in 21.3 % of them. Immune-mediated TEAEs grade ≥ 3 occurred in 5 % of patients.
Overall, Tislelizumab monotherapy showed a clinically meaningful and durable efficacy across several tumor types. This treatment was well tolerated, and no new safety signals were detected. From the researchers’ point of view, tislelizumab might be a potential new treatment option for MSI-H/dMMR solid tumors.
Figure 2: Best change in target lesion size from baseline by independent review committee (IRC)
HLX10: the upcoming treatment alternative across tumor types
Whereas MSI-H/dMMR advanced solid tumors have a poor prognosis when treated with conventional chemotherapy, they usually reach a high response to immune checkpoint inhibitors [6, 15, 16]. The investigational anti-PD-1 monoclonal antibody HLX10 – also known as serplulimab – has shown antitumor activity and a favorable safety and tolerability profile in preclinical and early clinical studies [17]. The ongoing single-arm, open-label, multicenter, phase II study (NCT03941574) evaluates the efficacy and safety of HLX10 monotherapy for the treatment of patients with histologically or cytologically confirmed unresectable or metastatic MSI-H/dMMR solid tumors who had progressed on or been intolerant to at least one prior standard therapy [18]. The patients receive an intravenous infusion of HLX10 (3 mg/kg) every two weeks for up to two years until disease progression, unacceptable toxicity, or withdrawal of informed consent.
By the time of the analysis (January 9, 2021), 108 patients were enrolled and 68 subjects with confirmed MSI-H were included in the main efficacy analysis. Median age of the patients was 53 years (range, 23-72). MSI-H tumor types included CRC (77.9 %), endometrial cancer (7.4 %), and gastric cancer (5.9 %). The most common prior treatments were oxaliplatin (83.8 %) and capecitabine (70.6 %). About 44.1 % patients were positive for programmed death-ligand 1 (PD-L1) at baseline.
After a median follow-up of 7.7 months, ORR per RECIST v1.1 – as assessed by an independent radiological review committee (IRRC) – and defined in the study protocol as the primary endpoint – achieved 38.2 % (95 % CI, 26.7-50.8; 2 CRs, 24 PRs and 20 SDs) and IRRC-DCR reached 67.6 % (95 % CI, 55.2-78.5). The ORR in the PD-L1 negative population (n = 29) and PD-L1 positive population (n = 30) amounted to 34.5 % and 46.7 %, respectively. Concerning the secondary endpoints reported, median DoR, PFS and OS were not attained, but a 12-month IRRC-assessed PFS and OS of 61.9 % (95 % CI, 49.0-72.5) and 81.2 % (95 % CI, 67.8-89.4) were respectively observed.
About half of the study population (49.1 %) experienced grade ≥ 3 TEAEs, primarily anemia (8.3 %), progressive disease (PD) (6.5 %), increased γ- glutamyltransferase (5.6 %) and intestinal obstruction (5.6 %). Immune-related adverse events (irAEs) appeared in 48.1 % of patients (grade ≥ 3 in 9.3 % of them). Altogether, three fatal cases (2.8 %; 2 PDs and 1 intestinal obstruction) possibly related to the investigational drug were reported.
The authors concluded that through its antitumor activity and its manageable safety profile, HLX10 has the potential to improve patients’ clinical outcomes as an effective and safe tissue-agnostic treatment.
Dostarlimab in MSI-H/dMMR tumors
Endometrial cancers (ECs) – the most commonly diagnosed gynecologic malignancy – is usually detected in early stages of the disease [19]. However, in 21 % of cases, EC has already spread to regional lymph nodes, and distant metastases are present at initial presentation in 9 % of patients [20]. Chemotherapy remains the standard treatment, despite modest efficacy; therefore, there is a high unmet therapeutic need in advanced EC [20].
Dostarlimab is a novel humanized anti-PD-1 monoclonal antibody developed for the treatment of various tumor types. Based on preliminary results of the GARNET trial (NCT02715284), dostarlimab has been recently approved in the EU and USA as monotherapy for the treatment of adult patients with dMMR recurrent or advanced EC which were progressive on or after a platinum-based regimen [20, 21].
The open-label, multicenter, single-arm phase I, ongoing GARNET study evaluates dostarlimab in patients with advanced solid malignant entities in several cohorts. Preliminary data showed a meaningful and durable clinical activity of dostarlimab in dMMR EC patients [18, 20]. At ASCO 2021, an interim analysis presented the results from cohort A1 (patients with advanced or recurrent dMMR/MSI-H EC) and cohort F (patients with dMMR or POLE-mutated non-EC solid tumors) individually and combined [22]. Dostarlimab was administered at 500 mg every three weeks for the first four cycles, and thereafter at 1000 mg every six weeks until disease progression or discontinuation.
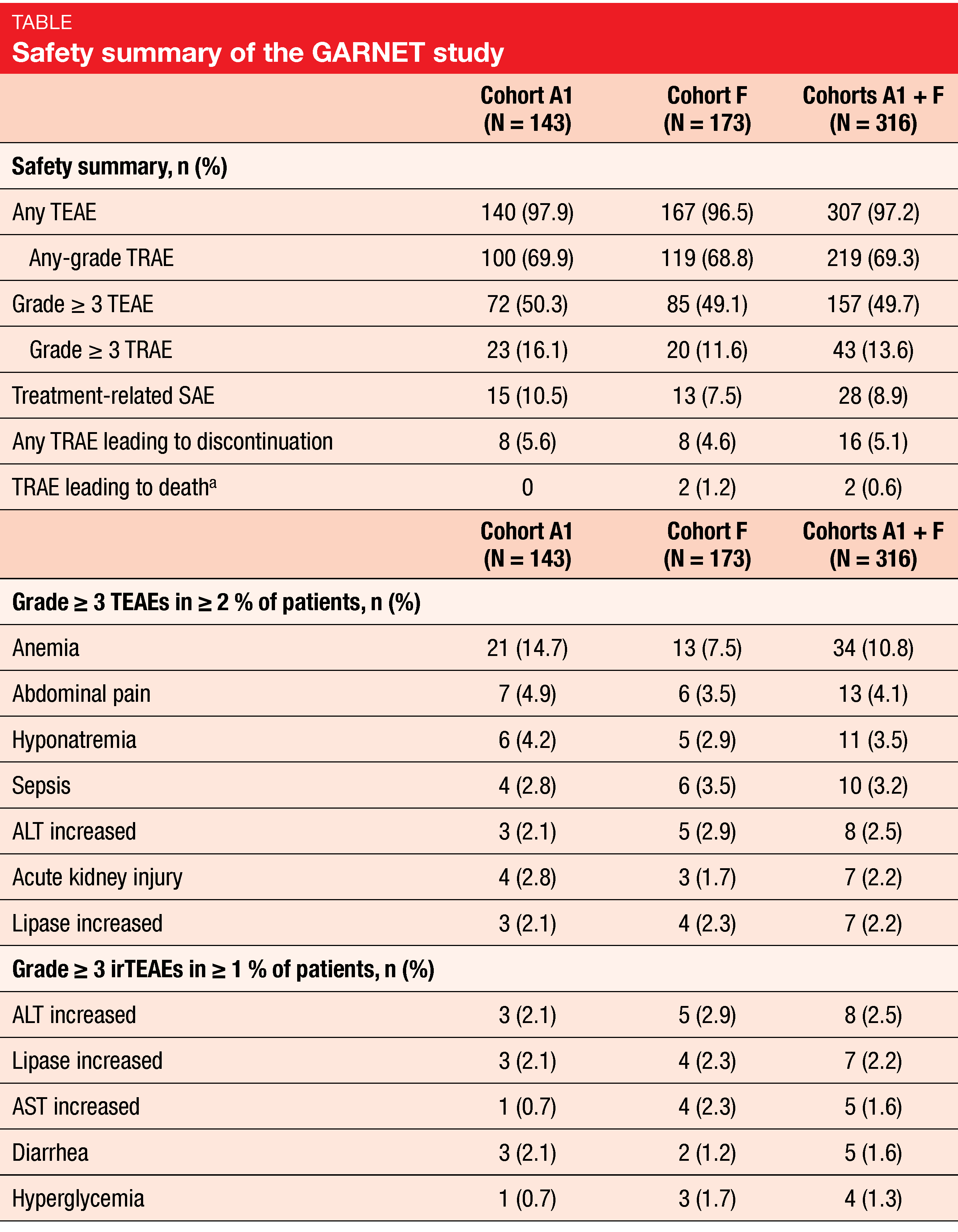
For this interim analysis, an efficacy analysis was performed for the patients who had baseline measurable disease and ≥ 6 months of follow-up in the study. Among those 209 patients in cohorts A1 + F, the median age of the patients was 63 years (A1, n = 103; F, n = 106). The cohorts A1 + F enclosed 103 patients with endometrial cancer, 69 patients with colorectal cancer, twelve patients with small-intestine cancer, as well as eight patients with gastric and gastroesophageal junction cancer and 17 patients with other non-EC tumor types. Three or more prior therapies were received by 10.7 % of patients in cohort A1, 31.1 % in cohort F, and 21.1 % in cohorts A1 + F. ORR amounted to 44.7 % (A1; 95 % CI, 34.9-54.8), 38.7 % (F; 95 % CI, 29.4-48.6), and 41.6 % (A1 + F; 95 % CI, 34.9-48.6), respectively. In total, eleven patients (10.7 %) reached a CR and 35 patients (34.0 %) a PR in cohort A1, eight CRs (7.5 %) and 33 PRs (31.1 %) in cohort F, and in total 19 CRs (9.1 %) and 68 PRs (32.5 %) in cohorts A1 + F. In combined cohorts, responses were durable (median DoR, 34.7 months; range, 2.6-35.8) and DCR was 60.3 % (range, 53.3-67.0).
Dostarlimab was well tolerated. In cohorts A1 + F, the most common grade ≥3 TEAEs were anemia (10.8 %), abdominal pain (4.1 %), hyponatremia (3.5 %) and sepsis (3.2 %), while immune-related TEAEs grade ≥3 experienced by 2.5 % of patients were increased alanine aminotransferase (ALT) lipase (2.2 %), respectively (Table). No deaths related to dostarlimab occurred.
In their conclusions, the authors reported an antitumor activity of dostarlimab in patients with different dMMR advanced or recurrent solid malignant entities, especially here in EC and non-EC cases. With mostly low-grade TREAS observed, dostarlimab showed a good tolerable safety profile across different tumor types.
REFERENCES
- Eso Y et al., Microsatellite instability and immune checkpoint inhibitors: toward precision medicine against gastrointestinal and hepatobiliary cancers. J Gastroenterol 2020; 55(1): 15-26.
- Cohen AC et al., Novel Therapeutics for Recurrent Cervical Cancer: Moving Towards Personalized Therapy. Drugs 2020; 80(3): 217-227.
- Marabelle A et al., Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 2020; 38(1): 1-10.
- Hargadon KM et al., Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol 2018; 62: 29-39.
- Le DT et al., PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015; 372(26): 2509-20.
- Lemery S et al., First FDA Approval Agnostic of Cancer Site – When a Biomarker Defines the Indication. N Engl J Med 2017; 377(15): 1409-1412.
- Marabelle A et al., Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020; 21(10): 1353-1365.
- Maio M et al., Pembrolizumab in microsatellite instability high (MSI-H)/mismatch repair deficient (dMMR) cancers: Updated analysis from phase 2 KEYNOTE-158 study. J Clin Oncol 2021; 39(suppl 15; abstr 2565).
- Zhang T et al., The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother 2018; 67(7): 1079-1090.
- Desai J et al., Phase IA/IB study of single-agent tislelizumab, an investigational anti-PD-1 antibody, in solid tumors. J Immunother Cancer 2020; 8(1): e000453.
- Shen L et al., Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study. J Immunother Cancer 2020; 8(1): e000437.
- Song Y et al., Treatment of relapsed or refractory classical Hodgkin lymphoma with the anti-PD-1, tislelizumab: results of a phase 2, single-arm, multicenter study. Leukemia 2020; 34(2): 533-542.
- Lee A et al., Tislelizumab: First Approval. Drugs 2020; 80(6): 617-624.
- Li J et al., A phase 2 study of tislelizumab monotherapy in patients with previously treated, locally advanced unresectable or metastatic microsatellite instability-high/mismatch repair deficient solid tumors. J Clin Oncol 2021; 39(suppl 15; abstr 2569).
- Le DT et al., Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017; 357(6349): 409-413.
- Overman MJ et al., Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017; 18(9): 1182-1191.
- Chao T et al., A novel anti-PD-1 antibody HLX10 study led to the initiation of combination immunotherapy. Ann Oncol 2019; 30(suppl 9): ix107-ix114.
- Qin S et al., Efficacy and safety of HLX10, a novel anti-PD-1 antibody, in patients with previously treated unresectable or metastatic microsatellite instability-high or mismatch repair-deficient solid tumors: A single-arm, multicenter, phase 2 study. J Clin Oncol 2021; 39(suppl 15; abstr 2566).
- Siegel RL et al., Cancer statistics, 2019. CA Cancer J Clin 2019; 69(1): 7-34.
- Kasherman L et al., Dostarlimab in the treatment of recurrent or primary advanced endometrial cancer. Future Oncol 2021; 17(8): 877-892.
- Markham A Dostarlimab: First Approval. Drugs 2021.
- Berton D et al., Antitumor activity of dostarlimab in patients with mismatch repair-deficient/microsatellite instability–high tumors: A combined analysis of two cohorts in the GARNET study. J Clin Oncol 2021; 39(suppl 15; abstr 2564).
© 2021 Springer-Verlag GmbH, Impressum
More posts
New therapeutic options being currently investigated in advanced or metastatic colorectal cancer
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States, and it is the fourth most frequent cancer diagnosis.A current treatment option for RAS and BRAF wild-type (WT) metastatic colorectal cancer (mCRC) is the chemotherapy doublet (FOLFOX/FOLFIRI) with an anti-EGFR monoclonal antibody (cetuximab or panitumumab).
An update and future directions in advanced gastric or gastrointestinal junction cancer (G/GEJC)
With more than 1 million newly diagnosed cases in 2020, gastric cancer (GC) is the fifth most frequent cancer; it was also the third leading cause of cancer-related death worldwide. Gastroesophageal junction (GEJ) cancer concerns a form of gastric cancer developing around the digestive tract where esophagus and stomach connect; in the last years, the prevalence of GEJ constantly increased.
Innovative combinations in esophageal squamous cell carcinoma
Each year, esophageal cancer (EC) is responsible for more than half a million deaths worldwide. Among them, esophageal squamous cell carcinoma (ESCC) accounts for the vast majority (~ 85 %) of EC incidences . At diagnosis, 70 % of ESCC is unresectable [3] and the 5-year survival rate is limited (30 % - 40 %). Patients with advanced or metastatic ESCC have a poor prognosis; their overall survival (OS) after standard first-line chemotherapy is limited to less than a year and other treatment options are scarce.
Novel agents or combinations in recurrent or metastatic nasopharyngeal cancer
Nasopharyngeal cancer (NPC) is a rare malignancy with an incidence of approximately 133,000 annually worldwide, resulting in about 80,000 deaths per year. Whereas early-stage and locally advanced NPC have a good prognosis, treatment of recurrent or metastatic nasopharyngeal cancer is a challenging; it is thus associated with a poor prognosis, especially in patients who have failed two or more lines of systemic therapy, with a median progression-free survival (mPFS) of seven months and median overall survival (mOS) of 22 months.
Preface ASCO Solid Tumor 2022
After 2 years of the COVID-19 pandemic, the Annual Meeting of the American Society of Clinical Oncology (ASCO), was held in Chicago, USA, and virtually from 3rd–7th June 2022.As always, the very much-anticipated event brought leading experts from across the globe together to learn and discuss the groundbreaking updates and scientific advancements which were covered in more than 2,000 abstracts, along with 85 livestream sessions, and more than 2,500 poster presentations.