PARP- and anti-PD-1-based strategies in breast and cervical cancer
Talazoparib in germline BRCA1/2-mutated breast cancer …
Breast cancer (BC) is the most diagnosed cancer in women and the leading cause of cancer death in females [1]. It has been recently shown that approximately 38 % of female patients younger than 40 years presenting with triple-negative breast carcinomas (TNBC) harbored a germline mutation in breast cancer (BC) susceptibility genes 1 or 2 (gBRCA1/2m) [2]. Treatment options are limited for patients with gBRCA1/2m BC, and the presence of these genetic alterations is associated with younger age at diagnosis, aggressive disease, dismal prognosis and higher risk of recurrence [3]. The enzyme poly (adenosine diphosphate-ribose) polymerase (PARP) plays an important role in the regulation of single-strand DNA breaks and PARP inhibitors induce tumor cell death due to accumulation of irreparable DNA damages [4]. Moreover, PARP inhibitors are well tolerated, oral targeted therapies [3]. Therefore, PARP inhibitors such as talazoparib are a welcome addition to the treatment arsenal for patients with gBRCA1/2m human epidermal growth factor receptor 2-negative (HER2-) locally advanced or metastatic BC [5]. Based on the findings of the pivotal phase III EMBRACA trial (NCT01945775), talazoparib received approval in October 2018 in the USA and in June 2019 in Europe for the treatment of adult patients with gBRCA1/2m, HER2-negative locally advanced or metastatic BC [5-7]. In this trial, talazoparib met its primary endpoint by showing a significantly better median progression-free survival (PFS) versus physician’s choice of chemotherapy (PCT) in this population (8.6 vs 5.6 months; HR, 0.54; 95 % CI, 0.41-0.71; p<0.001) [5]. A previous analysis of the EMBRACA trial investigating biomarkers associated with LONG and SHORT responders revealed that a tumor MYC amplification was associated with shorter overall survival (OS) in TNBC patients treated with talazoparib [8].
At ASCO virtual scientific meeting 2021, Ettl et al. reported about a retrospective post hoc analysis describing the clinical characteristics of LONG and SHORT responders following treatment with talazoparib or PCT in the EMBRACA study [9]. Patients in the intend-to-treat (ITT) population were mapped into two groups based on their response: LONG responders included patients with an OS ≥ 30 months and a duration of response (DOR) ≥ 24 months in the talazoparib arm (n=37) and an OS ≥ 30 months in the PCT arm (n=34); SHORT responders included patients in either arm with a PFS event ≤ 12 weeks (talazoparib arm, n=40; PCT arm, n=32). At the data cutoff date of September 30, 2019, a higher proportion of LONG responders with hormone receptor-positive (HR+) BC and no prior chemotherapy (CT) for locally advanced or metastatic BC was observed; additionally, a greater proportion of SHORT responders had TNBC and received ≥2 prior CT regimens for locally advanced or metastatic BC or platinum therapy. Approximately half of the LONG responders receiving talazoparib (51.4 %) and 91.2 % of the LONG responders treated with PCT had subsequent antineoplastic treatment. Moreover, at data cut off, more LONG responders (43.2 %) under talazoparib were still on treatment compared to the PCT arm (2.9 %). The median treatment duration for LONG responders was 33.5 months in the experimental arm and 7.6 months in the control arm, whereas patients from the SHORT group responded only 2.0 months in the talazoparib arm and 1.4 months in the PCT arm. As these findings were based on a limited data set, further investigations with a larger number of patients in this setting might be warranted.
… and in somatic BRCA1/2-mutated breast cancer
International guidelines recommend the use of PARP inhibitors for patients with metastatic HER2-negative BC with gBRCA1/2m, who were pretreated with chemotherapy in the neoadjuvant, adjuvant or metastatic stetting [10]. Only 5-10 % of BC are presenting gBRCA1/2 mutations and the current clinical usability of PARP inhibitors is limited so far to this population [11]. This raises the question whether PARP inhibitors are similarly effective in patients with somatic BRCA1/2m HER2-negative locally advanced or metastatic BC. Somatic BRCA1/2m were detected in circulating cell-free DNA (cfDNA) in 13.5 % of patients with metastatic BC and preclinical models have shown that pathogenic somatic BRCA1/2 mutations are sensitive to the PARP inhibitor talazoparib [12].
An ongoing multicenter, single-arm, phase II study (NCT03990896) has been initiated with the aim of evaluating the efficacy of talazoparib in patients with somatic BRCA1/2m metastatic BC detectable in cfDNA [13]. Eligible patients may have TNBC (with ≥ 1 prior CT) or HR+/HER2- BC (with ≥ 1 prior hormone therapy). Patients who received platinum therapy as neoadjuvant or adjuvant treatment, will have to observe at least a 6-month interval before being eligible for this trial. Patients must have adequate organ function, ECOG performance status ≤ 2 and should be PARP inhibitor naive. Patients receive talazoparib (1mg daily) until disease progression or intolerability; additionally, they undergo serial imaging using chest/abdomen/pelvis CT and bone scan at baseline and every twelve weeks, and as well as cfDNA analysis every four weeks. PFS by RECIST v1.1 was defined as the primary endpoint, while secondary endpoints include objective response rate (ORR) and safety assessed according to NCI CTCAE v5.0. Currently, two patients are completing screening at Massachusetts General Hospital (USA), where the study is already open; six more academic US centers will follow soon.
Durable clinical activity of pamiparib in HER2- BC
Pamiparib is an orally administered investigational selective PARP1/2 inhibitor, which demonstrated antitumor activity and was generally well tolerated in patients with advanced solid tumors [2, 4]. Preclinical models showed a good bioavailability and blood-brain penetration [14]. A single-arm, open-label, multi-center Chinese phase II study (NCT03575065) was designed to assess the efficacy and safety of pamiparib in patients diagnosed with locally advanced or metastatic HER2-negative BC, with deleterious or suspected deleterious gBRCA1/2m TNBC or HR+/HER2-, who received ≤ 2 prior line of chemotherapy [15]. Patients received pamiparib 60 mg orally twice daily in 28-day cycles. The primary endpoint of the trial was ORR per RECIST v1.1 assessed by an independent review committee (IRC); secondary endpoints included investigator-assessed OR (INV-ORR), DOR, best overall response (BOR), PFS, clinical benefit rate (CBR), disease control rate (DCR) and OS, as well as safety and tolerability.
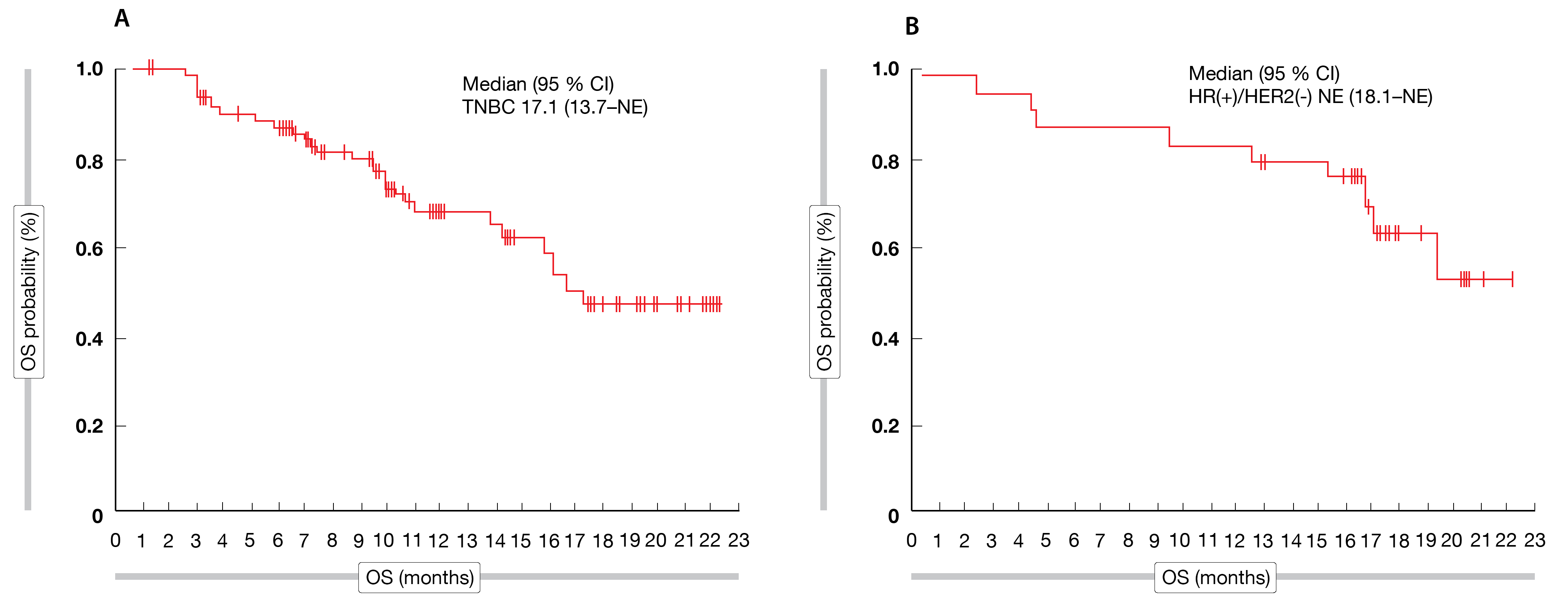
Out of 88 patients enrolled, 76 cases (55 in TNBC cohort and 21 in HR+ cohort) had measurable disease at baseline. The median age of patients was 46 years (range 27-67); 48 % were previously treated with platinum. The median study follow-up was 13.8 months (TNBC cohort: 10.9 months, HR+ cohort: 18.5 months). In the TNBC cohort, treatment with pamiparib demonstrated a confirmed ORR of 38.2 % (95 % CI: 25.4–52.3) and responses lasted for a median of seven months (95 % CI: 3.9–not estimable), while median PFS reached 5.5 months (95 % CI: 3.7–7.3) and median OS 17.1 months (95 % CI: 13.7–not estimable) (Figure 1A).
In the HR+ cohort, median ORR amounted to 61.9% (95 % CI: 38.4–81.9), median DOR was 7.5 months (95 % CI: 5.6-14.8), median PFS attained 9.2 months (95 % CI: 7.4–11.9) and survival data had not reached maturity at the time of the analysis (not reached; 95 % CI 18.1– not estimable) (Figure 1B). Four patients achieved a complete response (CR), including three in the TNBC cohort and one patient in the HR+ cohort. Overall, 18 patients in the TNBC cohort and twelve patients in the HR+ cohort experienced a partial response (PR). As assessed by IRC, 72.7 % of patients (95 % CI: 59.0-83.9) in the TNBC cohort and 90.5 % (95 % CI: 69.6-98.8) in the HR+ cohort achieved disease control. Additionally, a CBR of 43.6 % (95 % CI: 30.3-57.7) and of 71.4 % (95 % CI: 47.8-88.7) was reached in the TNBC- and HR+ cohorts, respectively.
Pamiparib was generally well tolerated, with treatment-emergent adverse event (TEAEs) leading to dose interruption in two patients (2.3 %) and to reduction in 57 patients (64.8 %). The most common ≥ grade 3 TEAEs were hematologic events, including anemia (in 39.8 % of patients), decreased neutrophil count (29.5 %) and decreased white blood cell count (21.6 %). The encouraging data obtained in this phase 2 study suggested that pamiparib might be a feasible and tolerable treatment strategy for this population.
Figure 1: Median OS in the TNBC (A) and the HR+ (B) cohorts. Data cut-off October 9, 2020
TBCRC 050: niraparib combined with trastuzumab
HER2 is overexpressed in around 20-30 % of BC tumors [16]. In addition to its role in DNA damage repair, PARP1 has also been implicated in other cellular functions including co-activation of genes such as NF-κB, which regulate tumor proliferation and HER2 drug resistance. It has been shown previously that the
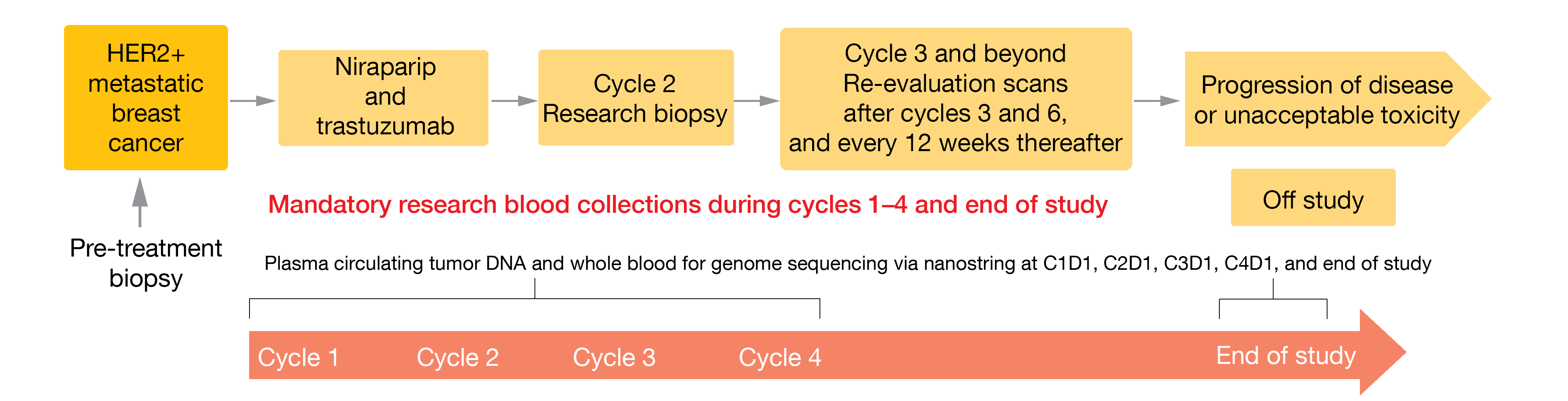
The TBCRC 050 trial is a multicenter, single-arm, phase Ib/II clinical study currently investigating the maximum tolerated dose and efficacy of the PARP inhibitor niraparib (200 mg orally) in combination with the anti-HER2 monoclonal antibody trastuzumab (6mg/kg, cycle 1 loading dose of 8 mg/kg) in patients diagnosed with locally advanced or metastatic unresectable HER2-positive BC (Figure 2) [18]. Eligible patients must have a measurable disease per RECIST v1.1 criterion, have already progressed under at least one prior HER2-targeted therapy, present a good performance status (ECOG PS 0-1) and a LVEF (left ventricular ejection fraction) ≥ 50 % by ECHO or MUGA, and have adequate bone-marrow, renal and liver functions. Patients initially treated with PARP inhibitors or having a concurrent endocrine therapy (for ER+/HER2+ patients) or having a known gBRCA1/2 BC are not eligible. The recruitment of patients with stable disease as well as treated CNS (central nervous system) metastases and/or carcinomatous meningitis is allowed. The primary objectives are to assess the dose-limiting toxicity (DLT) of this combined therapy, as well as the ORR. Blood and tissue biomarkers are collected to assess clinical benefit and to predict therapy response. Enrollment started in February 2021; this trial intends to recruit 40 patients in seven participating US sites.
Figure 2: TBCRC 050 study design: niraparib in combination with trastuzumab in patients with metastatic HER2-positive breast cancer
Synergy between anlotinib and sintilimab in cervical cancer
Cervical cancer is the fourth most common malignant disease in women with over 600,000 new diagnoses (6.5 %) per year worldwide and accounting for approximately 340,000 deaths (4th place) because of cancer yearly in this population [19]. Locally advanced or metastatic cervical cancer are associated with a chemotherapy with the option of adding the antiangiogenic agent bevacizumab; few treatment options exist in case of failure of this standard regimen [21]. As most cervical cancers have a viral etiology, which impairs the immune system, immune checkpoint inhibitors (CPIs) combined to other agents appears to be a promising strategy [20].
At the virtual ASCO 2021 meeting, Xu et al. presented the results of a new therapy combining anlotinib – a multi-target tyrosine kinase inhibitor inhibiting tumor angiogenesis and proliferative signaling – with sintilimab – a monoclonal antibody against programmed cell death-1 (PD-1) [22]. This single-arm, phase II Chinese study (ChiCTR1900023015) was conducted in patients with recurrent advanced cervical cancer to investigate the efficacy and safety of anlotinib plus sintilimab. Eligible patients should have received at least one prior platinum-based chemotherapy, have a good performance status (ECOG 0-1) and their tumor should show more than 1 % PD-L1 expression. Anlotinib was administered orally (10mg/day, d1-14, 21 days per cycle) and sintilimab intravenously (200mg once every 3 weeks). The primary endpoint was ORR, while DCR, PFS, OS and safety were the secondary endpoints.
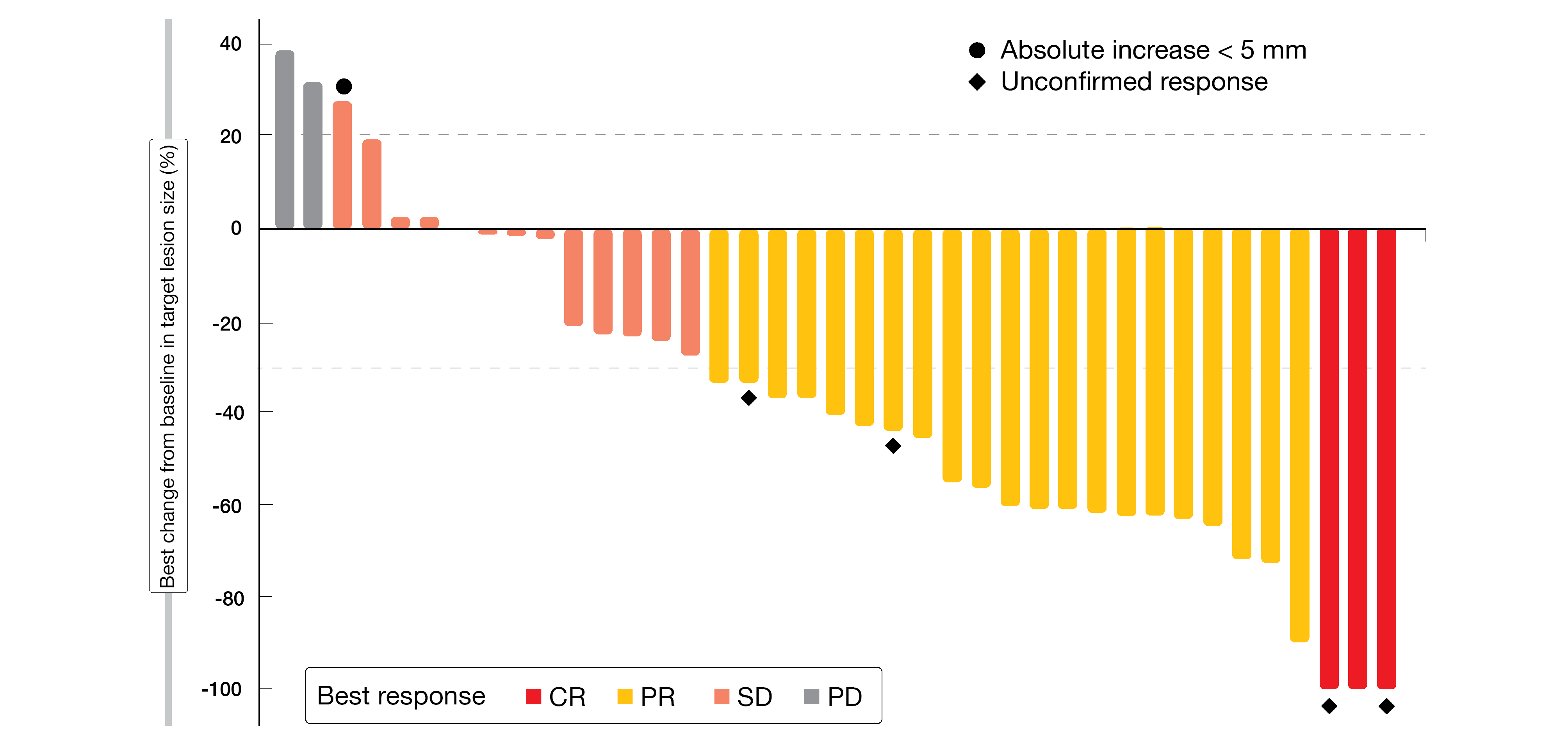
In total, 42 patients with a median age of 52 years (range, 47-58) were recruited. Among the 39 evaluable patients, objective response occurred in 61.5 % of patients (95 % CI, 44.9-75.9) and DCR was 94.9 % (95 % CI, 80.7-98.8). At the time of the analysis, the median PFS had not been reached yet. Three patients (8 %) achieved a CR and 21 (54 %) a PR, while 13 patients (33 %) had a stable disease (SD) (Figure 3). The most common adverse events (AEs) experienced by the patients were grade 1 or 2. Grade 3 AEs were hypertension (in 4.8 % of patients), hyponatremia (4.8 %), immune pneumonia (2.4 %) and immune myocarditis (2.4 %); no AEs grade 4 were observed. The authors pointed out that, according to the presented data, anlotinib plus sintilimab might represent a potential new treatment option with manageable safety profile in patients with recurrent advanced cervical cancer; they announced more data to be presented in the future.
Figure 3: Best response obtained following the combination therapy of anlotinib plus sintilimab
AdvanTIG-202: a novel combination in cervical cancer
Considering the elevated rate of PD-L1 expression in up to 80 % of cervical cancers [23], immune CPIs such as PD-1/PD-L1 inhibitors might be a novel therapeutic choice to improve clinical outcomes of patients with recurrent and/or metastatic cervical cancer. However, recent studies showed only moderate efficacy in this checkpoint inhibition. TIGIT (T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibition motif domain) is an co-inhibitory, immune checkpoint receptor, which is upregulated on T-cells and natural killer (NK) cells in various tumor cells [27]. Ociperlimab is a novel investigational anti-TIGIT monoclonal antibody. Tislelizumab – a human IgF4 monoclonal antibody binding to and blocking PD-1 receptor expressed on activated immune cells – has been approved in China in December 2019 and is involved in a broad clinical program combining various anti-cancer agents [28].
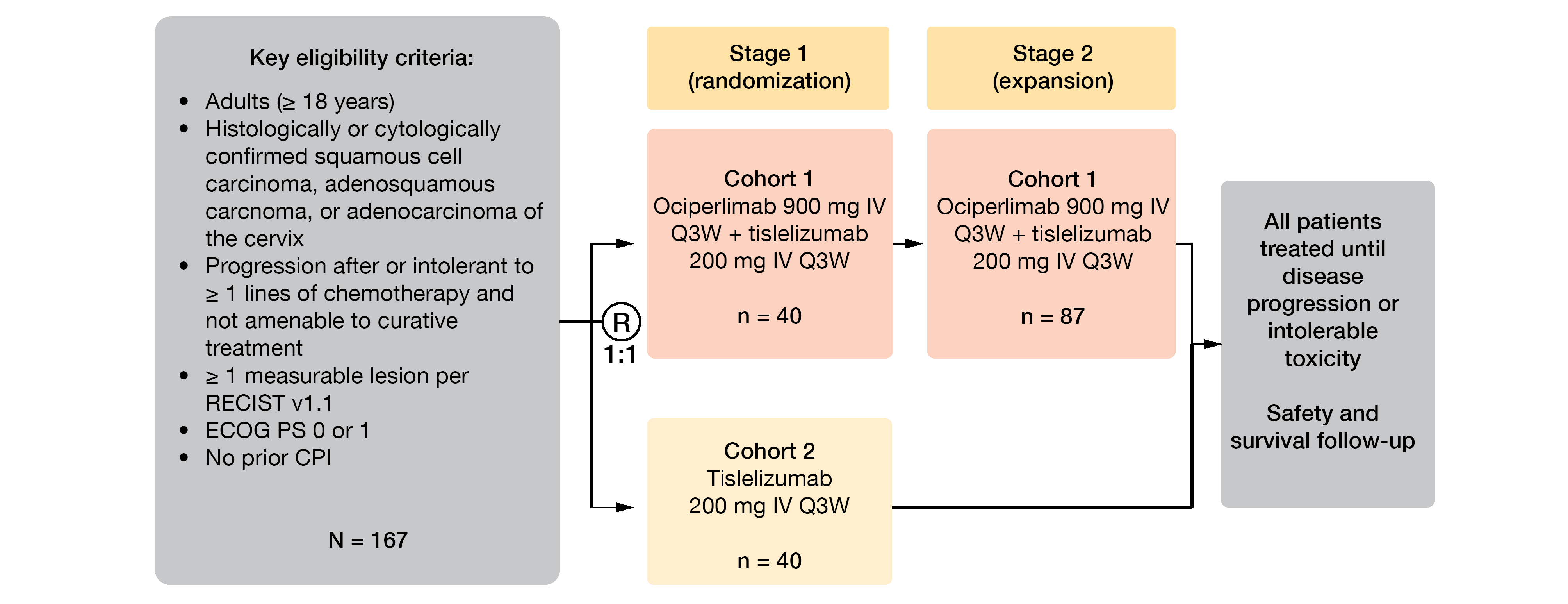
The multicenter, open-label, randomized, phase II study AdvanTIG-202 (NCT04693234) aim to investigate the clinical benefit of the addition of ociperlimab to tislelizumab. This trial will enroll in around 100 centers in Asia approximately 167 patients with cervical cancer (squamous cell carcinoma, adenosquamous carcinoma or adenocarcinoma) who progressed after at least one prior line of chemotherapy for recurrent or metastatic disease [22]. In Part 1, 80 patients will be randomized (1:1) to receive either ociperlimab (900 mg intravenously [IV]) in combination with tislelizumab (200 mg IV three-weekly) (Arm 1), or tislelizumab monotherapy (same dose than in Arm 1) in Arm 2, until disease progression, unacceptable toxicity, or withdrawal of consent (Figure 4). In Part 2, Arm 1 will be expanded by 87 additional patients whose tumors are evaluable for PD-L1 expression. ORR per RECIST v1.1 according to IRC constituted the primary endpoint, while secondary endpoints include investigator-assessed ORR, DOR, DCR, PFS, time to response (TTR), CBR, OS, safety, and tolerability; the exploratory endpoints are health-related quality of life (HR-QoL), as well as the association of biomarkers with patient prognosis and response, or tumor resistance.
Figure 4: Study design of AdvanTIG-202 study
REFERENCES
- Lickliter J et al., Dose Escalation/Expansion Study to Investigate the Safety, Pharmacokinetics, Food Effect, and Antitumor Activity of BGB-290 in Patients with Advanced Solid Tumors. Ann Onc 2017; 28 (suppl_5): v122-v141.
- Lupo B et al., Inhibition of poly(ADP-ribosyl)ation in cancer: old and new paradigms revisited. Biochim Biophys Acta 2014; 1846(1): 201-15.
- Cortesi L et al., An Overview of PARP Inhibitors for the Treatment of Breast Cancer. Target Oncol 2021; 16(3): 255-282.
- Xu B et al., Pamiparib dose escalation in Chinese patients with non-mucinous high-grade ovarian cancer or advanced triple-negative breast cancer. Cancer Med 2021; 10(1): 109-118.
- Litton JK et al., Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med 2018; 379(8): 753-763.
- Hoy SM, Talazoparib: First Global Approval. Drugs 2018; 78(18): 1939-1946.
- SmPC talazoparib Available from: https://www.ema.europa.eu/en/documents/product-information/talzenna-epar-product-information_en.pdf.
- Ettl J et al., Poster presented at SABCS virtual symposium 2020, San Antonio, Texas.
- Ettl J et al., Characterization of long-term responders following treatment with talazoparib (TALA) or physician’s choice of chemotherapy (PCT) in the phase 3 embraca trial. J Clin Oncol 2021; 39(suppl 15; abstr 1029).
- NCCN, Breast Cancer. 2021; Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419.
- Exman P et al., Evidence to date: talazoparib in the treatment of breast cancer. Onco Targets Ther 2019; 12: 5177-5187.
- Vidula N et al., Tumor Tissue- versus Plasma-based Genotyping for Selection of Matched Therapy and Impact on Clinical Outcomes in Patients with Metastatic Breast Cancer. Clin Cancer Res 2021; 27(12): 3404-3413.
- Vidula N et al., Phase II multicenter study of talazoparib for somatic BRCA1/2 mutant metastatic breast cancer. J Clin Oncol 2021; 39(suppl 15; abstr TPS1110).
- Xiong Y et al., Pamiparib is a potent and selective PARP inhibitor with unique potential for the treatment of brain tumor. Neoplasia 2020; 22(9): 431-440.
- Sun T et al., A phase 2 study of pamiparib in the treatment of patients with locally advanced or metastatic HER2-negative breast cancer with germline BRCA mutation. J Clin Oncol 2021; 39(suppl 15; abstr 1087).
- Mitri Z et al., The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy. Chemotherapy Research and Practice 2012; 2012: 743193.
- Stanley J et al., PARP1 and phospho-p65 protein expression is increased in human HER2-positive breast cancers. Breast Cancer Research and Treatment 2015; 150(3): 569-579.
- Stringer-Reasor EM et al., Trial in progress: A phase 1b/2 study of the PARP inhibitor niraparib in combination with trastuzumab in patients with metastatic HER2+ breast cancer (TBCRC 050). J Clin Oncol 2021; 39(suppl 15; abstr TPS1098).
- Sung H et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71(3): 209-249.
- Cohen AC et al., Novel Therapeutics for Recurrent Cervical Cancer: Moving Towards Personalized Therapy. Drugs 2020; 80(3): 217-227.
- Redondo A et al., Primary results from CECILIA, a global single-arm phase II study evaluating bevacizumab, carboplatin and paclitaxel for advanced cervical cancer. Gynecol Oncol 2020; 159(1): 142-149.
- Wu L et al., AdvanTIG-202: A phase 2 study investigating anti-TIGIT monoclonal antibody ociperlimab plus anti-PD-1 monoclonal antibody tislelizumab in patients with previously treated recurrent or metastatic cervical cancer. J Clin Oncol 2021; 39(suppl 15; abstr TPS5595).
- Duranti S et al., Role of Immune Checkpoint Inhibitors in Cervical Cancer: From Preclinical to Clinical Data. Cancers (Basel) 2021; 13(9).
- Liu Y et al., PD-1/PD-L1 Inhibitors in Cervical Cancer. Front Pharmacol 2019; 10: 65.
- Chung HC et al., Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 2019; 37(17): 1470-1478.
- Naumann RW et al., Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results From the Phase I/II CheckMate 358 Trial. J Clin Oncol 2019; 37(31): 2825-2834.
- Harjunpää H et al., TIGIT as an emerging immune checkpoint. Clin Exp Immunol 2020; 200(2): 108-119.
- Lee A et al., Tislelizumab: First Approval. Drugs 2020; 80(6): 617-624.
© 2021 Springer-Verlag GmbH, Impressum