Innovative combinations in esophageal squamous cell carcinoma
Each year, esophageal cancer (EC) is responsible for more than half a million deaths worldwide. Among them, esophageal squamous cell carcinoma (ESCC) accounts for the vast majority (~ 85 %) of EC incidences [1, 2]. At diagnosis, 70 % of ESCC is unresectable [3] and the 5-year survival rate is limited (30 % – 40 %) [4]. Patients with advanced or metastatic ESCC have a poor prognosis; their overall survival (OS) after standard first-line chemotherapy is limited to less than a year [5, 6] and other treatment options are scarce.
Expanded analysis of the CheckMate 648 study
On one hand, PD-L1 overexpression has been shown to be significantly associated with poor clinical outcome in ESCC patients [7], whereas on the other hand, the combination of chemotherapy with an immune checkpoint inhibitor (ICI) has demonstrated synergistic antitumoral activity [8]. In the CheckMate 648 study, nivolumab (NIVO) plus chemotherapy and NIVO plus ipilimumab (IPI) showed a significant better OS and a longer duration of response (DoR) compared to chemotherapy alone in therapy-naïve ESCC patients with PD-L1 expression ≥ 1 %, as well as in all randomized patients [9]. The expanded efficacy and safety analyses of Checkmate 648 were presented at this year’s ASCO meeting [10].
CheckMate 648 (NCT03143153) is a global, randomized, open-label phase III study, which investigates the efficacy and safety of nivolumab (anti-PD-1) as first-line therapy in patients with unresectable, advanced, recurrent, or metastatic ESCC. A total of 970 eligible patients were randomized following a 3-arm design (1:1:1) to receive either nivolumab (240 mg, every 2nd week [Q2W] plus chemotherapy (CT, fluorouracil + cisplatin, Q4W), or nivolumab (3 mg/kg, Q2W) plus the anti-CTLA-4 ICI ipilimumab (1 mg/kg, Q6W), or chemotherapy alone until disease progression, discontinuation due to toxicity or withdrawal. The co-primary endpoints were OS and progression-free survival (PFS) according to a blinded independent central review (BICR) in patients whose tumor cells expressed ≥1 % PD-L1. The secondary endpoints included OS and PFS in all randomized patients, as well as objective response rate (ORR), time to second objective disease progression (PFS2), duration of response (DoR) and safety.
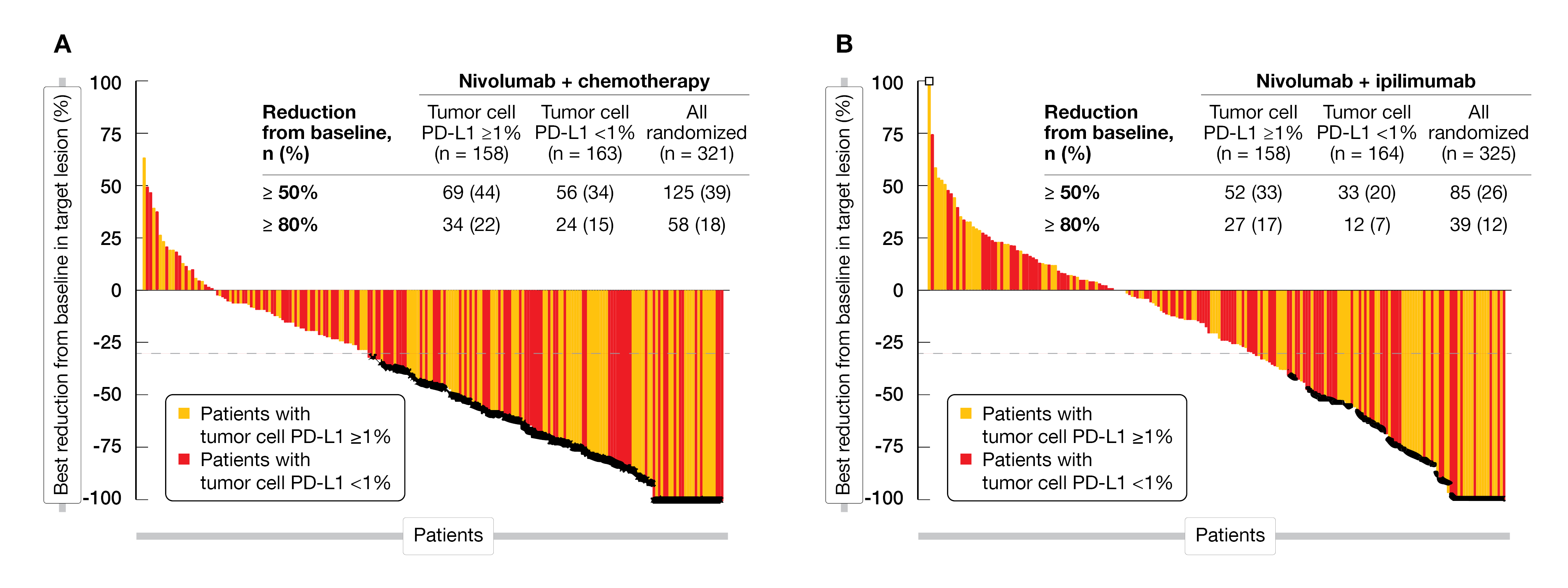
After a minimum of 12.9 months follow-up, the combination nivolumab plus chemotherapy showed a significant superior OS compared to chemotherapy alone (15.4 vs 9.2 months; unstratified HR=0.55) in patients with tumor cells PD-L1 ≥ 1 % or in all randomized patients (13.2 vs 10.7 months; unstratified HR=0.74). A PFS2 benefit (11.0 vs 7.9 months; HR=0.64; 95 % CI, 0.54-0.77) was also observed in all randomized patients. The ORR was higher in patients with tumor cell PD-L1 ≥ 1 % (53 % vs 20 %) or in all randomized patients (47 % vs 27 %) in the combination arm compared to the chemotherapy alone. Additionally, among all analyzed patient groups, a larger number of responders who received NIVO plus chemotherapy versus chemotherapy had a DoR of at least twelve months. The best percentage reduction from baseline in target lesion with NIVO plus chemotherapy is shown in Figure 1A.
Superior OS with NIVO plus IPI versus chemo was observed in patients with tumor cell PD-L1 ≥ 1 % (13.7 vs 9.2 months; unstratified HR=0.63) with no further enrichment in higher tumor cell PD-L1 expression subgroups; or in all randomized patients (12.7 vs 10.7 months; unstratified HR= 0.78) or related to the PFS2 (9.7 vs 7.9 months; HR=0.74) in all patients. Deep responses were observed with NIVO plus IPI compared to the chemotherapy, especially in patients with tumor cell PD-L1 ≥ 1 % (ORR, 35 % vs 20 %), while the DoR reached 11.8 versus 5.7 months, respectively (11.1 vs 7.1 months in all randomized patients). Figure 1B shows the best response in target lesion in this study arm.
No new safety signals were identified with both combinations (NIVO + chemotherapy or NIVO + IPI). Grade 3 and 4 treatment-related adverse events (TRAEs) with potential immunologic etiology occurred in ≤ 2 % of patients treated with NIVO plus chemotherapy and in ≤ 6 % of those who received NIVO plus IPI, with the majority of non-endocrine TRAEs being resolved in most patients following established adverse event management.
The authors concluded that these results further support NIVO plus chemotherapy and NIVO plus IPI as new 1L standard-of-care therapies for patients with advanced ESCC, especially for those having PD-L1 positive tumors.
Figure 1: Target lesion reduction in CheckMate 648 study (A) Nivolumab plus chemotherapy (B) Nivolumab plus ipilimumab. Best reduction is maximum reduction in sum of diameters of target lesions. Horizontal reference line indicates the 30 % reduction consistent with a response per RECIST v1.1. Asterix symbol represents responders. Square symbol represents percent change truncated to 100 %.
NXCEL1311 phase III study with nimotuzumab versus placebo
As approximately half of ESCC patients overexpress the epidermal growth factor receptor (EGFR), the antitumoral activity of nimotuzumab – an novel anti-EGFR monoclonal antibody – has been investigated in several clinical studies; these trials confirmed the antiproliferative, antiangiogenic and proapoptotic activity of nimotuzumab which enhances the sensitivity of certain solid tumors to chemotherapy and radiotherapy [11-13].
A currently ongoing, prospective, randomized, double-blind, multicenter, phase III study (NXCEL1311, NCT02409186) is investigating the efficacy and safety of nimotuzumab plus concurrent chemoradiotherapy (Nimo+CCRT) versus placebo plus chemoradiotherapy (Placebo+CCRT) in unresectable, locally advanced ESCC. In total, 200 eligible patients were randomized (1:1) to receive either nimotuzumab (400 mg, IV, D1, weekly) or placebo, both in combination with concurrent chemotherapy (paclitaxel [45 mg/m2, IV, D1, weekly], cisplatin [20 mg/m2, IV, D1, weekly]) for seven weeks and radiotherapy [3DCRT/IMRT: 59.4 Gy/33 times]). The OS was the primary endpoint, whereas PFS, ORR, disease control rate (DCR) and quality of life (QoL) were secondary endpoints.
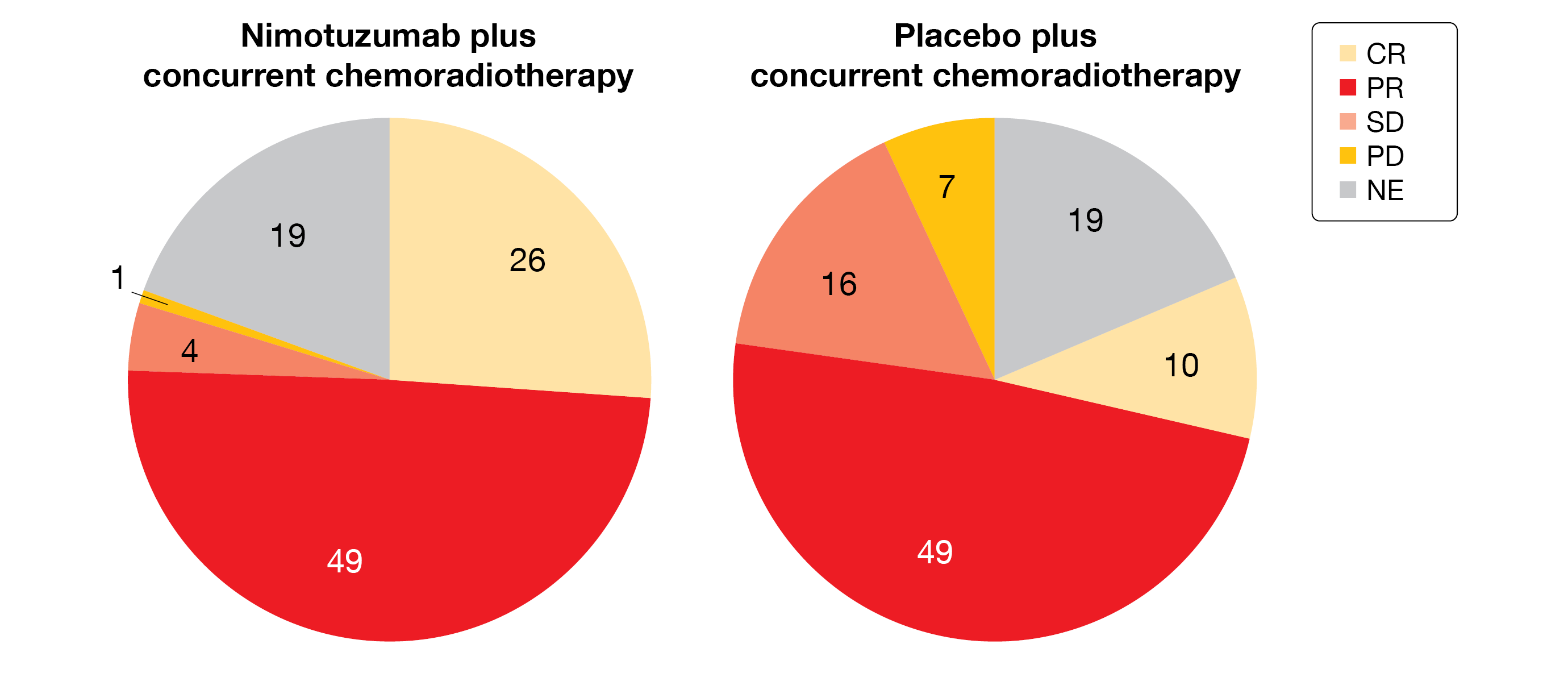
At ASCO 2022 meeting, the response rate (interim analysis) was presented [14]. Of the 80 evaluable patients in the Nimo+CCRT arm 26 patients achieved a complete response (CR) compared to 10 out of 82 evaluable patients in the Placebo+CCRT arm (CR-rate, 32.5 % vs 12.2 %; p=0.002); 49 patients had a partial response (PR) in both groups, while four patients reached a stable disease (SD) in the Nimo+CCRT arm (versus 16 patients in the Placebo+CCRT arm) (Figure 2). The ORR was significantly higher in the investigational group: 93.8 % in Nimo+CCRT arm versus 72.0 % in Placebo+CCRT arm (p<0.001). The DCR reached 98.8 % compared to 91.5 % (p=0.064), respectively. A single factor logistic regression evaluation showed that none of the factors analyzed (age, sex, target lesion number and BMI) did affect the efficacy outcome parameters (ORR, CR and DCR).
Overall, the incidence of grade ≥3 adverse drug reactions (ADRs) with Nimo+CCRT was comparable to those observed in the Placebo+CCRT arm (11.0 % vs 10.9 %; p>0.05); the most frequent grade ≥3 ADRs being leucopenia, bone marrow inhibition, fever, infectious pneumonia, nausea, neutropenia, and nutritional anemia.
The interim-analysis demonstrated promising efficacy and safety; a follow-up of five years is planned to finally analyze the effect on OS.
Figure 2: Response rate in both study arms of the phase III NXCEL1311 study.
Rationale 302: 2L tislelizumab versus chemotherapy
Tislelizumab – an IgG4 monoclonal antibody against PD-1 – has been designed to overcome resistance to anti-PD-1 therapy [15]. Its efficacy has been previously shown in multiple malignancies, including ESCC [16]. The primary analysis of the study data of the global phase III study RATIONALE 302 (NCT03430843) has already been presented at the ASCO 2021 meeting [17]; this trial investigated the efficacy and safety of tislelizumab compared to chemotherapy in patients with histologically confirmed unresectable advanced or metastatic ESCC, who progressed during or after a prior systemic therapy. The study met its primary endpoint as tislelizumab showed a statistically significant OS benefit compared to chemotherapy in the intent-to-treat population (ITT) (8.6 vs 6.3 months; HR=0.70; 95 % CI, 0.57-0.85; p=0.0001). At this year’s ASCO meeting, the outcomes of the Asia subgroup (China, Taiwan, Japan, and Korea) of the RATIONALE 302 trial have been reported [18].
Following disease progression after first-line systemic therapy, eligible Asian patients (404 out of 512 patients, 79 %) randomized 1:1 to receive tislelizumab (n=201; 200 mg, IV, Q3W) or chemotherapy (n=203) (paclitaxel, docetaxel, or irinotecan) until disease progression, intolerable toxicity, or withdrawal. The OS in all randomized patients was the primary endpoint, whereas the key secondary endpoints comprised OS in patients with PD-L1 Tumor Area Positivity Score ≥ 10 %; other secondary endpoints included PFS, ORR, DoR, health-related quality of life and safety.
After a median follow-up of 6.9 months, tislelizumab showed a significant improvement in OS compared to chemotherapy (8.5 vs 6.3 months; HR=0.73; 95 % CI, 0.59-0.90) in the Asia subgroup, while the median PFS reached 1.5 months with tislelizumab versus 1.7 months in the comparator arm (HR=0.81; 95 % CI, 0.64-1.02). Tislelizumab-treated patients had a higher ORR (20.4 % vs 9.4 %) and a longer DoR (7.4 vs 4.0 months; HR=0.42; 95 % CI, 0.21-0.84) versus chemotherapy.
Fewer TRAEs (74.1 % vs 95. 3 %), grade ≥3 TRAEs (19.4 % vs 57.1 %), serious TRAEs (15.4 % vs 20.9 %) and a similar proportion of grade 5 TRAEs (2.5 % vs 2.6 %) were reported with tislelizumab compared to chemotherapy.
The Asia subgroup results obtained with tislelizumab are consistent with the outcomes in the overall population; thus, tislelizumab is an efficient and safe second-line therapy option for patients with unresectable advanced or metastatic ESCC.
Rationale 302: health-related quality of life
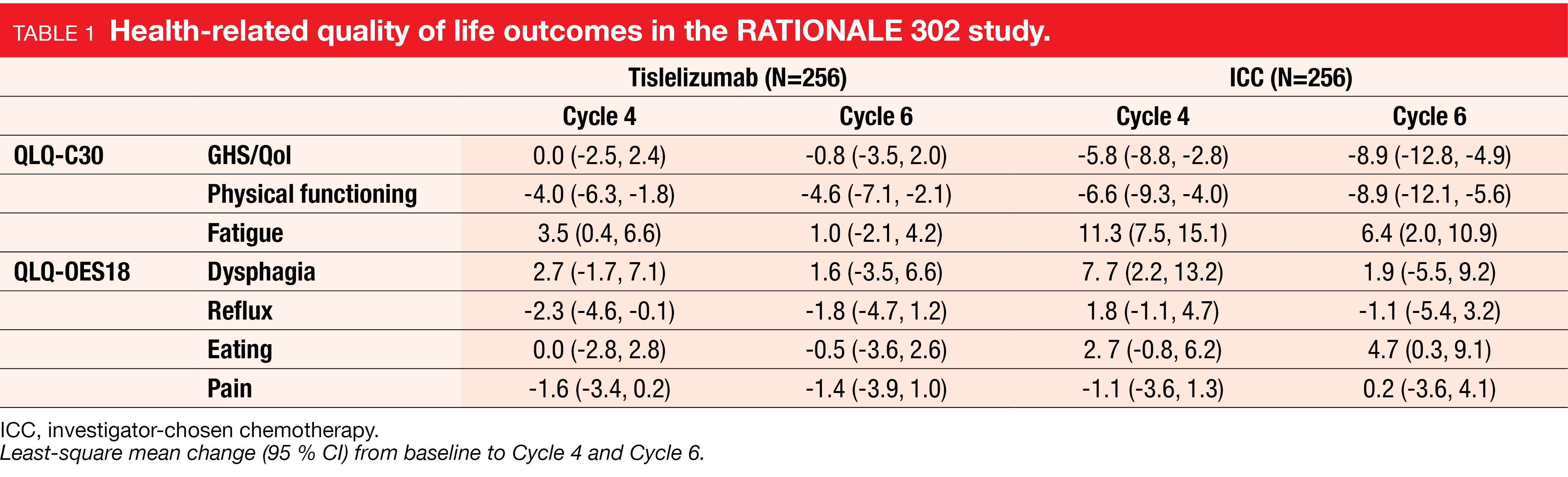
In the RATIONALE 302 study described above, the impact on health-related quality of life (HRQoL) was additionally evaluated by using the global health status/quality of life (GHS/QoL) questionnaire for measuring physical functioning, the EORTC QLQ-C30 for fatigue scores and the EORTC QLQ-OES18 for dysphagia, reflux, eating, and pain scores from screening visit through Cycle 6 or until treatment discontinuation (whichever occurred first) [19].
At Cycle 4 and Cycle 6, patients who were administered tislelizumab showed stable GHS/QoL and fatigue scores, as well as less decline in physical functioning compared to those who received chemotherapy (Cycle 4, -4.0 vs -6.6; Cycle 6, -4.6 vs -8.9, respectively) (Table 1). Patients treated with tislelizumab experienced less OES18 symptoms (except for pain) relative to baseline compared to those who received chemotherapy. The time to deterioration (TTD) for the GHS/QoL score – analyzed using the Kaplan-Meier method – showed that patients in the tislelizumab versus chemotherapy group had a lower risk for dysphagia worsening (HR=0.76; 95 % CI, 0.53-1.07; p=0.0562).
These data highlighted a longer maintenance of HRQoL for patients treated with tislelizumab compared to those who received chemotherapy. Taken together with the clinical outcomes of the RATIONALE 302 study, tislelizumab has great potential as new second-line treatment option for patients with advanced or metastatic ESCC.
Camrelizumab combined with fluorouracil as first-line therapy
In the treatment of ESCC, ICIs given as monotherapy did not show substantive improvements in terms of ORR and OS in patients with advanced ESCC [20]. As first-line therapy of ESCC, different combinations of ICIs with chemotherapy led to positive outcomes in several clinical trials (KEYNOTE-590, Checkmate-648, ORIENT-15 or ESCORT-1st) [21]. More than half of all ESCC cases worldwide are observed in China [22]; therefore, it is of interest to evaluate antitumoral activity and safety of such ICI/chemotherapy combination in the Chinese population.
Thus, a multicenter, open-label, prospective cohort study (ChiCTR2000037942) has been performed in China between May 2020 to February 2022 to evaluate the efficacy and safety of camrelizumab (anti-PD-1) combined with either fluorouracil or taxol/platinum [23]. In total, 40 patients with locally progressed and advanced ESCC have been enrolled in this trial to receive six cycles of camrelizumab plus chemotherapy (11 patients received fluorouracil/platinum and 29 taxol/platinum), followed by camrelizumab monotherapy.
After analysis of the first 33 patients (82.5 %), with a median treatment time of 5.8 months, the ORR reached 72.7 % and the DCR 97.0 %. No statistically significant difference was observed between both chemotherapy regimens in terms of ORR (55.6 % with fluorouracil/platinum vs 79.2 % with taxol/platinum; p=0.1779) or DCR (88.9 % vs 100 %, respectively; p=0.1005). Partial response was seen in 24 patients (19 with fluorouracil/platinum, 5 with taxol/platinum) and eight patients had a SD (5 vs 3 patients, respectively). At the time of this analysis, the median PFS has not been reached.
The most frequent grade 3 or 4 toxicities were thrombocytopenia, neutropenia, and leukopenia (2.5 % each). The most common immune-related AEs were reactive cutaneous capillary endothelial proliferation (12.5 %) and hypothyroidism (7.5 %). No new significant AEs were reported.
The authors concluded that camrelizumab plus chemotherapy is a promising regimen with good tolerability in the first-line treatment of ESCC.
1L lenvatinib plus pembrolizumab plus chemotherapy in ESCC
In the KEYNOTE-590, the benefit of pembrolizumab plus chemotherapy (5-fluorouracil [5-FU] + cisplatin) over chemotherapy alone has previously been shown as 1L treatment for unresectable locally advanced, recurrent, or metastatic esophageal cancer [24]. Lenvatinib is a multiple tyrosine kinase inhibitor against vascular endothelial growth factor receptor 1 (VEGFR1), VEGFR2 and VEGFR3. In combination with pembrolizumab, lenvatinib already showed promising antitumoral activity in advanced solid tumors [25-27].
The LEAP-014 trial (NCT04949256) is a randomized, 2-part, open-label, phase III study that aims to investigate the efficacy and safety of upfront lenvatinib plus pembrolizumab plus chemotherapy versus pembrolizumab plus chemotherapy in patients with metastatic ESCC [28]. The primary endpoint in part-1 of the study (Figure 3) is safety per NCI CTCAE v5.0 and tolerability (dose-limiting toxicity, DLT), whereas the dual primary endpoint in part-2 consists of OS and PFS by BICR per RECIST v1.1; the secondary endpoints include ORR by BICR per RECIST v1.1, DoR, and HRQoL.
In part-1 (safety run-in) of the study, six patients will receive an induction with intravenous pembrolizumab (400 mg, Q6W) for 2 cycles plus oral lenvatinib (8 mg, QD) plus intravenous chemotherapy (5-FU, 4000 mg/m2 on Day 1-Day 5, plus cisplatin, 80 mg/m2) for four cycles, then pembrolizumab (400 mg, Q6W for ≤16 doses) plus lenvatinib (20 mg, QD) for consolidation and are then closely monitored for 21 days after the first dose of study intervention for DLTs. In part-2 (main study), approximatively 850 adult patients with a histologically or cytologically confirmed metastatic ESCC, a measurable disease according to RECIST v1.1 and a good performance status (ECOG PS, 0 or 1) will be randomized (1:1) to receive either pembrolizumab plus lenvatinib plus chemotherapy (5-FU + cisplatin, IV, Q3W for 4 cycles or mFOLFOX6, Q2W for 6 cycles) followed by consolidation with pembrolizumab plus lenvatinib (arm A) or pembrolizumab plus chemotherapy (arm B), illustrated in Figure 3. The randomization will be stratified by PD-L1 combined positive score (≥10 vs < 10), region (East Asia vs North America and Western Europe vs rest of world), and chemotherapy backbone (5-FU plus cisplatin vs mFOLFOX6). The patients will be treated until progressive disease or unacceptable toxicity. This study is currently enrolling patients.
Figure 3: Study design of the LEAP-014 trial.
REFERENCES
- Arnold, M, et al., Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut 2020; 69(9): 1564-1571.
- Sung, H, et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71(3): 209-249.
- Arnold, M, et al., Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020; 159(1): 335-349.e15.
- Murphy, AG, et al., State of the art management of metastatic gastroesophageal cancer. Ann Transl Med 2015; 3(16): 236.
- Hiramoto, S, et al., A retrospective analysis of 5-fluorouracil plus cisplatin as first-line chemotherapy in the recent treatment strategy for patients with metastatic or recurrent esophageal squamous cell carcinoma. Int J Clin Oncol 2018; 23(3): 466-472.
- Moehler, M, et al., Cisplatin and 5-fluorouracil with or without epidermal growth factor receptor inhibition panitumumab for patients with non-resectable, advanced or metastatic oesophageal squamous cell cancer: a prospective, open-label, randomised phase III AIO/EORTC trial (POWER). Ann Oncol 2020; 31(2): 228-235.
- Wang, Q, et al., PD-L1 Expression On tumor Cells Was Associated With Unfavorable Prognosis In Esophageal Squamous Cell Carcinoma. J Cancer 2018; 9(12): 2224-2231.
- Baba, Y, et al., Tumor immune microenvironment and immune checkpoint inhibitors in esophageal squamous cell carcinoma. Cancer Sci 2020; 111(9): 3132-3141.
- Doki, Y, et al., Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med 2022; 386(5): 449-462.
- Chau, I, et al., Nivolumab (NIVO) plus chemotherapy (chemo) or ipilimumab (IPI) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): Expanded efficacy and safety analyses from CheckMate 648. J Clin Oncol 2022; 40(suppl 16; Abstr 4035).
- Crombet-Ramos, T, et al., Antiproliferative, antiangiogenic and proapoptotic activity of h-R3: A humanized anti-EGFR antibody. Int J Cancer 2002; 101(6): 567-575.
- You, B, et al., A dose-escalation phase I trial of nimotuzumab, an antibody against the epidermal growth factor receptor, in patients with advanced solid malignancies. Invest New Drugs 2011; 29(5): 996-1003.
- Zhao, L, et al., Nimotuzumab promotes radiosensitivity of EGFR-overexpression esophageal squamous cell carcinoma cells by upregulating IGFBP-3. J Transl Med 2012; 10: 249.
- Meng, X, et al., Nimotuzumab plus concurrent chemo-radiotherapy versus chemo-radiotherapy in unresectable locally advanced esophageal squamous cell carcinoma (ESCC): Interim analysis from a prospective, randomized-controlled, double-blinded, multicenter, and phase III clinical trial (NXCEL1311 Study). J Clin Oncol 2022; 40(suppl 16; Abstr 4016).
- Zhang, T, et al., The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother 2018; 67(7): 1079-1090.
- Shen, L, et al., Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study. J Immunother Cancer 2020; 8(1): e000437.
- Shen, L, et al., RATIONALE 302: Randomized, phase 3 study of tislelizumab versus chemotherapy as second-line treatment for advanced unresectable/metastatic esophageal squamous cell carcinoma. J Clin Oncol 2021; 39(suppl 15; abstr 4012).
- Zhao, K, et al., Randomized, phase 3 study of second-line tislelizumab vs chemotherapy in advanced or metastatic esophageal squamous cell carcinoma, RATIONALE 302: Asia subgroup. J Clin Oncol 2022; 40(suppl 16; Abstr e16107).
- Van Cutsem, E, et al., Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma (ESCC, RATIONALE 302): Impact on health-related quality of life (HRQoL). J Clin Oncol 2022; 40(suppl 16; Abstr 16095).
- Kato, K, et al., Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20(11): 1506-1517.
- Thuss-Patience, P, et al., Immunotherapy in Squamous Cell Cancer of the Esophagus. Curr Oncol 2022; 29(4): 2461-2471.
- Hou, H, et al., Survival of Esophageal Cancer in China: A Pooled Analysis on Hospital-Based Studies From 2000 to 2018. Front Oncol 2019; 9: 548.
- Zhao, J, et al., Camrelizumab in combination with fluorouracil or taxol plus platinum chemotherapy as first-line treatment of esophageal squamous cell carcinoma: A multicenter, open-label, prospective cohort study. J Clin Oncol 2022; 40(suppl 16; Abstr e16084).
- Sun, JM, et al., Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 2021; 398(10302): 759-771.
- Finn, RS, et al., Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol 2020; 38(26): 2960-2970.
- Makker, V, et al., Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N Engl J Med 2022; 386(5): 437-448.
- Motzer, R, et al., Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med 2021; 384(14): 1289-1300.
- Sun, J, et al., First-line lenvatinib plus pembrolizumab plus chemotherapy in esophageal squamous cell carcinoma: LEAP-014 trial in progress. J Clin Oncol 2022; 40(suppl 16; Abstr TPS4167).
© 2022 Springer-Verlag GmbH, Impressum
More posts
New therapeutic options being currently investigated in advanced or metastatic colorectal cancer
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States, and it is the fourth most frequent cancer diagnosis.A current treatment option for RAS and BRAF wild-type (WT) metastatic colorectal cancer (mCRC) is the chemotherapy doublet (FOLFOX/FOLFIRI) with an anti-EGFR monoclonal antibody (cetuximab or panitumumab).
An update and future directions in advanced gastric or gastrointestinal junction cancer (G/GEJC)
With more than 1 million newly diagnosed cases in 2020, gastric cancer (GC) is the fifth most frequent cancer; it was also the third leading cause of cancer-related death worldwide. Gastroesophageal junction (GEJ) cancer concerns a form of gastric cancer developing around the digestive tract where esophagus and stomach connect; in the last years, the prevalence of GEJ constantly increased.
Innovative combinations in esophageal squamous cell carcinoma
Each year, esophageal cancer (EC) is responsible for more than half a million deaths worldwide. Among them, esophageal squamous cell carcinoma (ESCC) accounts for the vast majority (~ 85 %) of EC incidences . At diagnosis, 70 % of ESCC is unresectable [3] and the 5-year survival rate is limited (30 % - 40 %). Patients with advanced or metastatic ESCC have a poor prognosis; their overall survival (OS) after standard first-line chemotherapy is limited to less than a year and other treatment options are scarce.
Novel agents or combinations in recurrent or metastatic nasopharyngeal cancer
Nasopharyngeal cancer (NPC) is a rare malignancy with an incidence of approximately 133,000 annually worldwide, resulting in about 80,000 deaths per year. Whereas early-stage and locally advanced NPC have a good prognosis, treatment of recurrent or metastatic nasopharyngeal cancer is a challenging; it is thus associated with a poor prognosis, especially in patients who have failed two or more lines of systemic therapy, with a median progression-free survival (mPFS) of seven months and median overall survival (mOS) of 22 months.
Preface ASCO Solid Tumor 2022
After 2 years of the COVID-19 pandemic, the Annual Meeting of the American Society of Clinical Oncology (ASCO), was held in Chicago, USA, and virtually from 3rd–7th June 2022.As always, the very much-anticipated event brought leading experts from across the globe together to learn and discuss the groundbreaking updates and scientific advancements which were covered in more than 2,000 abstracts, along with 85 livestream sessions, and more than 2,500 poster presentations.